|
|
|
- しょうこ かみいしづ
- 5 years ago
- Views:
Transcription
1
2
3
4
5 Table 1. Serum and tissue levels after a single oral administrsion of gatiflozacin ND: not detected(0. 05 ug/g or ug/eml) Table 2. Reason for exclusion and dropped out from evaluation
6 VOL.47 NO.10 Fig. 1. Number of patients evaluted. Table 3. Background
7 Table 4. Clinical efficacy by diagnosis Efficacy rate (%) =(excellent good) / No. of patients
8 Table 5. Clinical effcacy by daily dose (excellent good) Efficacy rate (%) = / No. of patients Table 6. Efficacy in radiological examination Improvement rate (%)= (remarkably improved+improved+slighty improved) / No. of patients
9 Table 7-1. Sensitivity distribution of clinical isolates (wind) MIC (ligiml: 10.CFU/m1) ylococcus aureus 0.39memL, coagulase negative staphylococci (CNS) 0.2 ggiml, Streptococcus pyo- genes 0.39 g/ml, Streptococcus pneumoniae 0.78 ge
10 Table 7-2. Sensitivity distribution of clinical isolates (jig/m1) MIC (mg/ml: 10TFU/m1) ml, Pseudomonas aeruginosa 3.13 it g/ml, Haemophi-
11 Table 8. Bacteriological effect Eradication rate (%) =eradicated + replaced / No. of patients - unassessable
12 VOL.47 NO.10 Table 9. Elimination rate by isolated organism
13
14 Table 12. Abnormal laboratory test findings Table 13. Usefulness
15 5) Nakashima M, Uematsu T, Kongo K, at AL; Single-. and multiple-dose pharmacokinetics of AM -1155, a new 6-fluoro-8-methoxy quinolone, in 1) Hosaka M, Yasue T, Fukuda H, et al.: In vitro and in vivo antibacterial activities of AM-1155, a new 6- fluoro-8 methoxy quinolone., Antimicrob. Agents Chemother. 36: , ) Wakabayashi E, Mitsuhashi S: In vitro antibacterial activity of AM-1155, a novel 6 fluoro-8- methoxy quinolone., Antimicrob. Agents Chemother. 38: , ) Hosaka M, Kinoshita S, Toyama A, et al.: Antibacterial properties of AM-1155, a new 8 methoxy humans. Antimicrob. Agents Chemother. 39: , ) Fukuda H, Hori S, Hiramatsu K: Antibacterial Activity of Gatifloxacin (AM-1155, CG 5501, BMS ), a Newly Developed Fluoroquinolone, against Sequentially Acquired Quinolone-Resistant Mutants and the nora Transformant of Staphylococcus aureus, Antimicrob. Agents Chemother. `
16 42: , ) Fukuda H, Hiramatsu K: Primary Targets of Fluoroquinolones in Streptococcus pneumoniae, Antimicrob. Agents Chemother. 43: , ) Tomizawa H, Tateda K, Miyazaki 8, et al.: Antibacterial activity of AM-1155 against penicillinresistant Streptococcus pneumoniae, J. Antimicrob. Chemother. 41: , 1998 Pharmacokinetic and clinical studies of gatifloxacin on otorhinolaryngological infections Shunkichi Baba1), Kenji Suzuki1), Ginichiro Ichikawa1), Takuya Yamakawa2), Ichiro Ando3), Takatsugu Itabashe, Makoto Sakai5), Atsushi Shinkawe, Hirosato Miyake6), Mutsumi Sate, Tadashi Akitaye, Akira Yokota1), Takehiro Kobayashi8), Satoaki Hojyo9), Isato Tsuge10), Toshio Yamashita1), Nobuo Kubo11), Yasuo Harada13), Koji YAjin12), Katsuhiro Hirakawa12), Nobuharu Tagashira13), Isao Nishida14), Ryo Omura14), Makoto Shirane16), Masahumi Nikaido15), Masaru Ohyama, Tetsuya Shima16), Takuo Nobori17), Ichiro Moriyama16), Kunihiko Sakamoto19), Masato Ushikail9), Hiromi Yam20) and Koichi Deguchi21) 1) Department of Otorhinolaryngology, Nagoya City University 1 Kawazumi, Mizuho-cho, Mizuho-ku, Nagoya 467, Japan, Medical School, Department of Otorhinolaryngology, Juntendo University, 2) School of Medicine Department of Otorhinolaryngology, Juntendo University Juntendo Urayasu 3) Hospital 4)Department of Otorhinola ryngology, Koto Hospital Department of Otorhinolaryngology, Tokai 5) University, School of Medicine Department of Otorhinolaryngology, Tokai University Tokyo Hospital 6) Department of Otorhinolaryngology, Tokai University Ohiso Hospital 7) Department of Otorhinolaryngology, Nagoya City Higashi Municipal 8) Hospital Department of Otorhinolaryngology, Kasugai Municipal Hospital Department of Otorhinolaryngology, Toyohashi Municipal 10) Hospital Department of Otorhinolaryngology, Kansai College of Medicine 11) Department of Otorhinolaryngology, Hiroshima University, 12) School of Medicine Department of Otorhinolaryngology, Hiroshima Red Cross and Atomic 13) Bomb Survivor Hospital Department of Otorhinolaryngology, W.F.A.C, Hiroshima General Hospital Department of Otorhinolaryngology, Hiroshima General Hospital of 15) West Japan Railway Company Department of Otorhinolaryngology, Kagoshima University, School of Medicine Department of Otorhinolaryngology, Imakiire General Hospital Department of Otorhinolaryngology, Kagoshima Prefectural 18) Hokusatsu Hospital Department of Otorhinolaryngology, Kagoshima Prefectural Oshima Hospital 19) 9) 17) 14) 16) Department of Otorhinolaryngology, Sendai Hospital Research Section, Tokyo Clinical Research Center 21) 20) Pharmacokinetic and clinical studies were carried out with gatifloxacin (GFLX) in otorhinolaryngological infections. The results were as follows: 1. In the middle ear mucosa, the concentration of GFLX ranged from undetectable levels (ND) to 0.66 /leg at 120 to 165 minutes after oral administration of 100 mg, and from ND to 1.11 pg/g at 140 to 510 minutes after oral administration of 200 mg, and the penetration ratio (tissue/serum) ranged from 0.23 to In the paranasal sinus mucosa, the concentration of GFLX ranged from 1.06 to 1.91 II g/g at 120 to 170 minutes after oral administration of 100 mg, and from 1.79 to 4.29 'leg at 110 to 160 minutes after oral administration of 200 mg, and the penetaration ratio ranged from 1.41 to In the paroid gland, the concentraion of GFLX ranged from 0.34 to 2.52 "Leg at 150 to 350 minutes after oral administration of 100 mg, and the penetration ratio ranged from 1.82 to 7.20.
17 2. The clinical efficacy rate was 75.7% (28/37) for otitis media, 88.2% (15/17) for paranasal sinusitis, 85.2% (23/27) for tonsillitis, 81.8% (9/11) for pharyngolaryngitis, 86.7% (13/15) for otitis externa, and 4/4 for suppurative sialadenitis. The overall clinical efficacy was excellent in 39, good in 53, fair in 14, and poor in 5, with the efficacy rate of 82.9% (92/111). 3. The elimination rate was 87.0% (67/77) for gram-positive aerobes, 90.9% (30/33) for gram-negative aerobes, and 100% (8/8) for anaerobes, and resulted in the overall rate of 89.0% (105/108). 4. Although adverse reactions were observed in 5 of 115 cases (4.3%) and abnormal laboratory test findings were recorded in 4 of 92 cases (4.3%), none were serious. These results indicate that GFLX is a very useful drug for the treatment of otorhinolaryngological infections.
 Table 1.Concentration of gatifloxacin (Middle-ear) Table 2.Concentration of gatifloxacin (Paranasal sinuses) Table 3.Concentration of gatifloxacin (Tonsil) Table 4.No.of patients studied Table 5.Background
Table 1.Concentration of gatifloxacin (Middle-ear) Table 2.Concentration of gatifloxacin (Paranasal sinuses) Table 3.Concentration of gatifloxacin (Tonsil) Table 4.No.of patients studied Table 5.Background
coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, F
 VOL.43 S-1 coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, Flavobacter- Table 1. Concentration of grepafloxacin
VOL.43 S-1 coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, Flavobacter- Table 1. Concentration of grepafloxacin
VOL. 34 S-2 CHEMOTH8RAPY 913
 VOL. 34 S-2 CHEMOTH8RAPY 913 914 CHEMOTHERAPY APR. 1986 Fig. 1 Chemical structure of T-2588 and T-2525 T- 2588 pivaloyloxymethyl (+ )- (6 R, 7 R)-7-[(Z)-2- (2-amino- 4-thiazolyl)-2-methox yiminoacetamido]-3-[(
VOL. 34 S-2 CHEMOTH8RAPY 913 914 CHEMOTHERAPY APR. 1986 Fig. 1 Chemical structure of T-2588 and T-2525 T- 2588 pivaloyloxymethyl (+ )- (6 R, 7 R)-7-[(Z)-2- (2-amino- 4-thiazolyl)-2-methox yiminoacetamido]-3-[(
 CHEMOTHERAPY APRIL 1992 VOL. 40 S- 1 Table 1-1. Comparative in vitro activity of meropenem against clinical isolates CNS: coagulase-negative staphylococci CHEMOTHERAPY APRIL 1992 Table 1-2. Comparative
CHEMOTHERAPY APRIL 1992 VOL. 40 S- 1 Table 1-1. Comparative in vitro activity of meropenem against clinical isolates CNS: coagulase-negative staphylococci CHEMOTHERAPY APRIL 1992 Table 1-2. Comparative
 Table 1-1. Criteria for evaluation Table 2. Criteria for bacteriological efficacy Table 1-2. Criteria for evaluation Table 3. Reasons for exclusion and drop-out from evaluation (clinical efficacy) Table
Table 1-1. Criteria for evaluation Table 2. Criteria for bacteriological efficacy Table 1-2. Criteria for evaluation Table 3. Reasons for exclusion and drop-out from evaluation (clinical efficacy) Table
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates
 Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
 CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study
 CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
VOL.42 S-1
 CHEMOTHERAPY APR. 1994 VOL.42 S-1 CHEMOTHERAPY APR. 1994 Table 1. Criteria for evaluation of clinical efficacy by the Japanese Society of Oral and Maxillo-Facial Surgeons Grades of symptoms and numerical
CHEMOTHERAPY APR. 1994 VOL.42 S-1 CHEMOTHERAPY APR. 1994 Table 1. Criteria for evaluation of clinical efficacy by the Japanese Society of Oral and Maxillo-Facial Surgeons Grades of symptoms and numerical
 Table 1.Quality control of MICs for reference strains Table 2.Antimicrobial activity of gatifloxacin against aerobic bacteria Table 4.Antimicrobial activity of gatifloxacin and other quinolones against
Table 1.Quality control of MICs for reference strains Table 2.Antimicrobial activity of gatifloxacin against aerobic bacteria Table 4.Antimicrobial activity of gatifloxacin and other quinolones against
CHEMOTHERAPY Fig. 1 Chemical structure of CXM-AX
 Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
VOL. 40 S- 1 Table 1. Susceptibility of methicillin-resistant Staphylococcus aureus to meropenem Table 2. Coagulase typing of methicillin-resistant St
 CHEMOTHERAPY VOL. 40 S- 1 Table 1. Susceptibility of methicillin-resistant Staphylococcus aureus to meropenem Table 2. Coagulase typing of methicillin-resistant Staphylococcus aureus CHEMOTHERAPY Table
CHEMOTHERAPY VOL. 40 S- 1 Table 1. Susceptibility of methicillin-resistant Staphylococcus aureus to meropenem Table 2. Coagulase typing of methicillin-resistant Staphylococcus aureus CHEMOTHERAPY Table
Fig.2. Sensitivity distribution of clinical isolates of S. epidermidis (24 strains, 106 CFU/ml) Staphylococcus aureus Staphylococcus epider- midis Ent
 Fig.2. Sensitivity distribution of clinical isolates of S. epidermidis (24 strains, 106 CFU/ml) Staphylococcus aureus Staphylococcus epider- midis Enterococcus faecalis Klebsiella pneumoniae, Morganella
Fig.2. Sensitivity distribution of clinical isolates of S. epidermidis (24 strains, 106 CFU/ml) Staphylococcus aureus Staphylococcus epider- midis Enterococcus faecalis Klebsiella pneumoniae, Morganella
Fig. 1 Chemical structure of DL-8280
 Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
CHEMOTHERAPY FEB Table 1. Activity of cefpirome and others against clinical isolates
 VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
CHEMOTHERAPY
 CHEMOTHERAPY VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin CHEMOTHERAPY AUG. 1993 VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin Table 1. Comparative in vitro activity of teicoplanin
CHEMOTHERAPY VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin CHEMOTHERAPY AUG. 1993 VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin Table 1. Comparative in vitro activity of teicoplanin
CHEMOTHERAPY aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Prote
 aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Proteus mirabilis 3.13, Proteus vulgaris 1.56, Citrobacter freundii 0.39,
aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Proteus mirabilis 3.13, Proteus vulgaris 1.56, Citrobacter freundii 0.39,
VOL.35 S-2 CHEMOTHERAPY Table 1 Sex and age distribution Table 2 Applications of treatment with carumonam Table 3 Concentration of carumonam in human
 CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
988 CHEMOTHERAPY NOV. 1971
 988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S.
 Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
366 12 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 Dec. 2012 1 8 DNA 2,3 16 12 20 171 2008 12 2010 11 2 3,558 4.44% 1.65% 1.17% 90% 9 Escherichia coli -
 Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 365 11 sita oxacin 1 1 1 1 1 1 2 2 3 3 1 1 1 2 3 2012 9 14 sita oxacin STFX 50 mg 10% 2008 1 2008 12 2010 11 2 STFX 1,452 91.4% 1,235/1,351 95.9% 466/486
Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 365 11 sita oxacin 1 1 1 1 1 1 2 2 3 3 1 1 1 2 3 2012 9 14 sita oxacin STFX 50 mg 10% 2008 1 2008 12 2010 11 2 STFX 1,452 91.4% 1,235/1,351 95.9% 466/486
VOL.32 S-7 CHEMOTHERAPY Table 1 MIC of standard strains of CTRX Fig. 2 Cumulative curves of MIC S. aureus (26 strains )
 CHEMOTHERAPY OCT. 1984 Fig. I Chemical structure of CTRX VOL.32 S-7 CHEMOTHERAPY Table 1 MIC of standard strains of CTRX Fig. 2 Cumulative curves of MIC S. aureus (26 strains ) CHEMOTHERAPY Fig. 3 Cumulative
CHEMOTHERAPY OCT. 1984 Fig. I Chemical structure of CTRX VOL.32 S-7 CHEMOTHERAPY Table 1 MIC of standard strains of CTRX Fig. 2 Cumulative curves of MIC S. aureus (26 strains ) CHEMOTHERAPY Fig. 3 Cumulative
CHEMOTHERAPY JUN Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter cloacae 27, Proteus rettgeri 7, Proteus inconstans 20, Proteus
 VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity of gram nega
 1066 CHEMOTHERAPY MAR. 1975 Table 1 Sensitivity of standard strains VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity
1066 CHEMOTHERAPY MAR. 1975 Table 1 Sensitivity of standard strains VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity
1272 CHEMOTHERAPY MAR. 1975
 1272 CHEMOTHERAPY MAR. 1975 VOL. 23 NO. 3 CHEMOTHERAPY 1273 Fig. 2 Minimal inhibitory concentration of aminoglycosides against 50 strains of Klebsiella Fig. 1 Minimal inhibitory concentration of aminoglycosides
1272 CHEMOTHERAPY MAR. 1975 VOL. 23 NO. 3 CHEMOTHERAPY 1273 Fig. 2 Minimal inhibitory concentration of aminoglycosides against 50 strains of Klebsiella Fig. 1 Minimal inhibitory concentration of aminoglycosides
CHEMOTHERAPY Table 1 Clinical effect of Sultamicillin
 CHEMOTHERAPY CHEMOTHERAPY Table 1 Clinical effect of Sultamicillin CHEMOTHERAPY Fig. 1 MICs of sultamicillin against respiratory pathogenic Branhamella catarrhalis 62 strains, inoculum size 106CFU/m1 Fig.
CHEMOTHERAPY CHEMOTHERAPY Table 1 Clinical effect of Sultamicillin CHEMOTHERAPY Fig. 1 MICs of sultamicillin against respiratory pathogenic Branhamella catarrhalis 62 strains, inoculum size 106CFU/m1 Fig.
CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels of mezlocillin
 CHEMOTHERAPY Fig. 2 Urinary excretion of mezlocillin Fig. 1 Blood levels of mezlocillin CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels
CHEMOTHERAPY Fig. 2 Urinary excretion of mezlocillin Fig. 1 Blood levels of mezlocillin CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels
CHEMOTHERAPY MAY. 1988
 CHEMOTHERAPY MAY. 1988 CHEMOTHERAPY Fig. 1 Chemical structure CHEMOTHERAPY MAY. 1988 VOL.36 5-1 CHEMOTHERAPY CHEMOTHERAPY MAY. 1988 VOL.36 S-1 CHEMOTHERAPY CHEMOTHERAPY MAY. 1988 VOL.36 S-1 CHEMOTHERAPY
CHEMOTHERAPY MAY. 1988 CHEMOTHERAPY Fig. 1 Chemical structure CHEMOTHERAPY MAY. 1988 VOL.36 5-1 CHEMOTHERAPY CHEMOTHERAPY MAY. 1988 VOL.36 S-1 CHEMOTHERAPY CHEMOTHERAPY MAY. 1988 VOL.36 S-1 CHEMOTHERAPY
CHEMOTHERAPY NOV S. aureus, S. epidermidis, E. coli, K. pgeumoniae, E. cloacae, S. marcescens, P. mirabilis, Proteus, P. aeruginosa Inoculum siz
 VOL.33 S-5 CHEMOTHERAPY 381 Fig. 1 Chemical structure of HAPA-B Chemical name 1-N-[(2S)-3-Amino-2-hydroxypropiony1]-4-0-(6-amino- 6-deoxy-a-D-glucopyranosyl)-6-013-deoxy-4-C-methyl- 3-(methylamino)-ƒÀ-L-arabinopyranosyl]-2-deoxystreptamine
VOL.33 S-5 CHEMOTHERAPY 381 Fig. 1 Chemical structure of HAPA-B Chemical name 1-N-[(2S)-3-Amino-2-hydroxypropiony1]-4-0-(6-amino- 6-deoxy-a-D-glucopyranosyl)-6-013-deoxy-4-C-methyl- 3-(methylamino)-ƒÀ-L-arabinopyranosyl]-2-deoxystreptamine
epidermidis, Enterococcus faecalis, Enterococcus Klebsiella pneumoniae, Proteus mirabilis, indolepositive Proteus spp., Enterobacter spp., Serratia
 epidermidis, Enterococcus faecalis, Enterococcus Klebsiella pneumoniae, Proteus mirabilis, indolepositive Proteus spp., Enterobacter spp., Serratia Table 3. Overall clinical efficacy of cefozopran in
epidermidis, Enterococcus faecalis, Enterococcus Klebsiella pneumoniae, Proteus mirabilis, indolepositive Proteus spp., Enterobacter spp., Serratia Table 3. Overall clinical efficacy of cefozopran in
Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone
 Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone (inoculum size= 106 CFU/ml) (Ĉ-lactamase producer : 2 strains) Fig. 1. Sensitivity distribution of
Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone (inoculum size= 106 CFU/ml) (Ĉ-lactamase producer : 2 strains) Fig. 1. Sensitivity distribution of
Staphylococcus sp. K.pneumoniae P.mirabilis C.freundii E. cloacae Serratia sp. P. aeruginosa ml, Enterococcus avium >100ƒÊg/ml
 CHEMOTHERAPY SEPT. 1992 cefoperazone ceftazidime (CAZ), imipenem (IPM) Staphylococcus sp., Enterococcus (CPZ), faecalis, Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii, Enterobacter cloacae,
CHEMOTHERAPY SEPT. 1992 cefoperazone ceftazidime (CAZ), imipenem (IPM) Staphylococcus sp., Enterococcus (CPZ), faecalis, Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii, Enterobacter cloacae,
 VOL.31 S-2 CHEMOT HERAPY 101 Table 1 Breakdown of cases Table 2 List of excluded cases Table 3 List of drop-out cases 102 CHEMOT HERAPY JULY 1983 Table 4 Background factors of suppurative otitis media
VOL.31 S-2 CHEMOT HERAPY 101 Table 1 Breakdown of cases Table 2 List of excluded cases Table 3 List of drop-out cases 102 CHEMOT HERAPY JULY 1983 Table 4 Background factors of suppurative otitis media
Table 1. Concentration of ritipenem in plasma, gallbladder tissue and bile after ritipenem acoxil administration (200 mg t.i.d., 3 days) N.D.: not det
 Table 1. Concentration of ritipenem in plasma, gallbladder tissue and bile after ritipenem acoxil administration (200 mg t.i.d., 3 days) N.D.: not detected *: time after last administration Table 2. Concentration
Table 1. Concentration of ritipenem in plasma, gallbladder tissue and bile after ritipenem acoxil administration (200 mg t.i.d., 3 days) N.D.: not detected *: time after last administration Table 2. Concentration
Fig. 1 Chemical structure of norfioxacin (AM-715)
 Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
Fig. 1 Chemical structure of KW-1070
 Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
CHEMOTHERAPY
 CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of Sulbactam/CPZ against standard strains MIC mg/ml Inoculum size 106 CFU/ml * Sulbactam/CPZ= 1: 1 ** Concentration of Sulbactam+ CPZ CHEMOTHERAPY
CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of Sulbactam/CPZ against standard strains MIC mg/ml Inoculum size 106 CFU/ml * Sulbactam/CPZ= 1: 1 ** Concentration of Sulbactam+ CPZ CHEMOTHERAPY
Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII (45)
 Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2305(45) 2306(46) THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 Dec. Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2307(47) 2308(48) THE JAPANESE
Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2305(45) 2306(46) THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 Dec. Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2307(47) 2308(48) THE JAPANESE
Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-
 Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl) oxy]-14-ethyl-12,
Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl) oxy]-14-ethyl-12,
 Table 1 Sensitivity distribution of clinical isolates 1. Escherichia coli Inoculum size: 106cells/ml 2. Klebsiella pneumoniae 3. Enterobacter cloacae 4. Serratia marcescens Inoculum size: 106cells/nil
Table 1 Sensitivity distribution of clinical isolates 1. Escherichia coli Inoculum size: 106cells/ml 2. Klebsiella pneumoniae 3. Enterobacter cloacae 4. Serratia marcescens Inoculum size: 106cells/nil
 Table 1 Method of administration Inactive placebo. Table 2 Critoria for overall ef4fficy rating by committee L Severity and scores of main symptoms 1) Acute tonsillitis (Peritonsillids, Peritonsillar abscess)
Table 1 Method of administration Inactive placebo. Table 2 Critoria for overall ef4fficy rating by committee L Severity and scores of main symptoms 1) Acute tonsillitis (Peritonsillids, Peritonsillar abscess)
CHEMOTHERAPY
 CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of BRL 28500 against standard strains of bacteria Fig, 1 Sensitivity distribution of ABPC-resistant E. coli isolated from urinary tract Fig. 2 Sensitivity
CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of BRL 28500 against standard strains of bacteria Fig, 1 Sensitivity distribution of ABPC-resistant E. coli isolated from urinary tract Fig. 2 Sensitivity
Fig.1 Chemical structure of BAY o 9867
 Fig.1 Chemical structure of BAY o 9867 CHEMOTHERAPY 43 Table 3 Antibacterial spectrum of gram negative bacteria Medium:Heart infusion agar (Nissui) Method:Agar dilution (Streak) CHEMOTHERAPY DEC 1985
Fig.1 Chemical structure of BAY o 9867 CHEMOTHERAPY 43 Table 3 Antibacterial spectrum of gram negative bacteria Medium:Heart infusion agar (Nissui) Method:Agar dilution (Streak) CHEMOTHERAPY DEC 1985
 VOL. 17 NO. 7 CHEMOTHERAPY 1305 1) W. BRumFirr et al. : Clinical and laboratory studies with carbenicillin. Lancet 1: 1289~ 1293, 1967 2) E. T. KNUDSEN et al. : A new semisynthetic penicillin active against
VOL. 17 NO. 7 CHEMOTHERAPY 1305 1) W. BRumFirr et al. : Clinical and laboratory studies with carbenicillin. Lancet 1: 1289~ 1293, 1967 2) E. T. KNUDSEN et al. : A new semisynthetic penicillin active against
pneumoniae 30, C. freundii 32, E. aerogenes 27, E. cloacae 32, P. mirabilis 31, P. vulgaris 34, M. morganii 32, S. marcescens 31, H. influenzae 27, P.
 pneumoniae 30, C. freundii 32, E. aerogenes 27, E. cloacae 32, P. mirabilis 31, P. vulgaris 34, M. morganii 32, S. marcescens 31, H. influenzae 27, P. aeruginosa 30, P. maltophilia pyogenes 32, Escherichia
pneumoniae 30, C. freundii 32, E. aerogenes 27, E. cloacae 32, P. mirabilis 31, P. vulgaris 34, M. morganii 32, S. marcescens 31, H. influenzae 27, P. aeruginosa 30, P. maltophilia pyogenes 32, Escherichia
 Table 1 Antibacterial spectra of CPM and other antimicrobials against anaerobes Fig. 1 In vitro activity of CPM and other antibiotics against B. fragilis (136 strains) Fig. 2 In vitro activity of CPM and
Table 1 Antibacterial spectra of CPM and other antimicrobials against anaerobes Fig. 1 In vitro activity of CPM and other antibiotics against B. fragilis (136 strains) Fig. 2 In vitro activity of CPM and
日本化学療法学会雑誌第59巻第5号
 Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
VOL.42 S-1 methicillin-susceptible Staphylococcus aureus (MSSA), Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Klebsiella pneum
 VOL.42 S-1 methicillin-susceptible Staphylococcus aureus (MSSA), Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae methicillin-resistant Staphylococcus
VOL.42 S-1 methicillin-susceptible Staphylococcus aureus (MSSA), Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae methicillin-resistant Staphylococcus
 Fig. 1 Chemical structure of norfloxacin Table 1. Institutes attended to the study The Department of Dermatology, Defense Medical College The Department of Dermatology, School of Medicine, Teikyo University
Fig. 1 Chemical structure of norfloxacin Table 1. Institutes attended to the study The Department of Dermatology, Defense Medical College The Department of Dermatology, School of Medicine, Teikyo University
 1) Chemical name: Fig. 1 Chemical structure of TE-031 (-)-(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2, PYranosyl)oxy]-14-ethyl-12,13-dihydroxy-7-meth 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexo- oxy-3,5,7,9,11,13-hexamethy1-6-[[3,4,6-trideoxy-3-
1) Chemical name: Fig. 1 Chemical structure of TE-031 (-)-(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2, PYranosyl)oxy]-14-ethyl-12,13-dihydroxy-7-meth 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexo- oxy-3,5,7,9,11,13-hexamethy1-6-[[3,4,6-trideoxy-3-
Table1MIC of BAY o 9867 against standard strains
 Table1MIC of BAY o 9867 against standard strains Fig.2Cumulative and Distribution Curves of MIC (S.aureus 54 strains) 106cfu/ml Fig.3Correlogram of MIC (S.aureus 54 strains) CHEMOTHERAPY 451 Fig.4Cumulative
Table1MIC of BAY o 9867 against standard strains Fig.2Cumulative and Distribution Curves of MIC (S.aureus 54 strains) 106cfu/ml Fig.3Correlogram of MIC (S.aureus 54 strains) CHEMOTHERAPY 451 Fig.4Cumulative
VOL.47 NO.5 Table 1. Susceptibility distribution of Ĉ- lactams against clinical isolates of MRSA MRSA: rnethicillin- resistant Staphylococcus aureus
 MAY 1999 VOL.47 NO.5 Table 1. Susceptibility distribution of ƒà- lactams against clinical isolates of MRSA MRSA: rnethicillin- resistant Staphylococcus aureus (oxacillin MIC: 4ƒÊg/ ml) FMOX: flomoxef,
MAY 1999 VOL.47 NO.5 Table 1. Susceptibility distribution of ƒà- lactams against clinical isolates of MRSA MRSA: rnethicillin- resistant Staphylococcus aureus (oxacillin MIC: 4ƒÊg/ ml) FMOX: flomoxef,
 b) Gram-negative bacteria Fig. 2 Sensitivity distribution of clinical isolates : E. coli Fig. 3 Sensitivity distribution of clinical isolates : Pseudomonas Fig. 1 Sensitivity distribution of clinical isolates
b) Gram-negative bacteria Fig. 2 Sensitivity distribution of clinical isolates : E. coli Fig. 3 Sensitivity distribution of clinical isolates : Pseudomonas Fig. 1 Sensitivity distribution of clinical isolates
CHEMOTHERAPY DEC (NFLX), ofloxacin (OFLX), ciprofloxacin (CPFX) Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecali
 CHEMOTHERAPY DEC. 1988 (NFLX), ofloxacin (OFLX), ciprofloxacin (CPFX) Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis Pseudomonas aeruginosa, Serratia ma- Fig. 1. Chemical
CHEMOTHERAPY DEC. 1988 (NFLX), ofloxacin (OFLX), ciprofloxacin (CPFX) Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis Pseudomonas aeruginosa, Serratia ma- Fig. 1. Chemical
THE JAPANESE JOURNAL OF ANTIBIOTICS 48-8 Enterococcus avium 5Š, Corynebacterium xerosis 10Š, Corynebacterium pseudodiphtheriticum 10Š, Corynebacterium
 THE JAPANESE JOURNAL OF ANTIBIOTICS 48-8 Enterobacter spp., Serratia spp., Burkholderia cepacia, Flavobacterium spp., Alcaligenes spp. THE JAPANESE JOURNAL OF ANTIBIOTICS 48-8 Enterococcus avium 5Š, Corynebacterium
THE JAPANESE JOURNAL OF ANTIBIOTICS 48-8 Enterobacter spp., Serratia spp., Burkholderia cepacia, Flavobacterium spp., Alcaligenes spp. THE JAPANESE JOURNAL OF ANTIBIOTICS 48-8 Enterococcus avium 5Š, Corynebacterium
400 46 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 Dec. 2012 LVFX 100 mg 3 / 7 150 mg 2 / 7 2 2006 2008 9 LVFX PK PD 2009 7 100 mg 1 3 500 mg 1 1 AUC/MIC
 Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
 VOL.30 NO.10 CHEMOTHERAPY 1123 Fig,1 Group B case 6 hepatolithiasis,e.k.66 y.0.,f.45kg Postoperative wound infection Fig.2 Group B case 15 gastric cancer,k.k.60 y.o.,m. Postoperative peritonitis Fig.3
VOL.30 NO.10 CHEMOTHERAPY 1123 Fig,1 Group B case 6 hepatolithiasis,e.k.66 y.0.,f.45kg Postoperative wound infection Fig.2 Group B case 15 gastric cancer,k.k.60 y.o.,m. Postoperative peritonitis Fig.3
 Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
 CHEMOTHERAPY Silver sulfadiazine (T 107) CHEMOTHERAPY Fig. 1 Item of patients Table 1 Criteria of bacteriological efficacy by the Committee xcellent: E Score of infection becomes to 0% at the end of medication.
CHEMOTHERAPY Silver sulfadiazine (T 107) CHEMOTHERAPY Fig. 1 Item of patients Table 1 Criteria of bacteriological efficacy by the Committee xcellent: E Score of infection becomes to 0% at the end of medication.
CHEMOTHERAPY SEPT. 1970
 CHEMOTHERAPY SEPT. 1970 VOL. 18 NO. 5 CHEMOTHERAPY CHEMOTHERAPY SEPT. 1970 VOL. 18 NO. 5 CHEMOTHERAPY 691 692 CHEMOTHERAPY SEPT. 1970 VOL. 78 NO. CHEMOTHERAPY 5 写 真1 第1病 693 写 真4 日 In vitroの しCEZの 第22病
CHEMOTHERAPY SEPT. 1970 VOL. 18 NO. 5 CHEMOTHERAPY CHEMOTHERAPY SEPT. 1970 VOL. 18 NO. 5 CHEMOTHERAPY 691 692 CHEMOTHERAPY SEPT. 1970 VOL. 78 NO. CHEMOTHERAPY 5 写 真1 第1病 693 写 真4 日 In vitroの しCEZの 第22病
CHEMOTHERAPY FEB Table 1 Background of volunteers
 CHEMOTHERAPY FEB. 1985 Table 1 Background of volunteers Table 3 Reproducibility of saisomicln In the EIA and the RIA Fig.1 Comparison of the EIA with the RIA or bioassay of sisomicin Table 4 Blood levels
CHEMOTHERAPY FEB. 1985 Table 1 Background of volunteers Table 3 Reproducibility of saisomicln In the EIA and the RIA Fig.1 Comparison of the EIA with the RIA or bioassay of sisomicin Table 4 Blood levels
日本化学療法学会雑誌第53巻第S-3号
 moxifloxacin in vitro moxifloxacin in vitro 17 9 6 17 11 21 moxifloxacinmflx in vitro cefdinir CFDNclavulanic acidamoxicillincvaampcclarithromycincamclindamycincldm levofloxacinlvfx 1MFLX Clostridium clostridiiformeclostridium
moxifloxacin in vitro moxifloxacin in vitro 17 9 6 17 11 21 moxifloxacinmflx in vitro cefdinir CFDNclavulanic acidamoxicillincvaampcclarithromycincamclindamycincldm levofloxacinlvfx 1MFLX Clostridium clostridiiformeclostridium
Table 1 Classification of female patients with vesical irritating symptom by their signs : Urinary pain with or without other vesical irritability. s
 Table 1 Classification of female patients with vesical irritating symptom by their signs : Urinary pain with or without other vesical irritability. s Vesical irritability without urinary Pain. Pyuria 10/
Table 1 Classification of female patients with vesical irritating symptom by their signs : Urinary pain with or without other vesical irritability. s Vesical irritability without urinary Pain. Pyuria 10/
 1.Streptococcus pneumoniae, Streptococcus pyogenes JC-1,S.aureus Smith,methicillin (DMPPC)- susceptible S. aureus subsp. aureus (MSSA) TR101, DMPPC-resistant S. aureus subsp. aureus (MRSA) TR102, Staphylococcus
1.Streptococcus pneumoniae, Streptococcus pyogenes JC-1,S.aureus Smith,methicillin (DMPPC)- susceptible S. aureus subsp. aureus (MSSA) TR101, DMPPC-resistant S. aureus subsp. aureus (MRSA) TR102, Staphylococcus
Clostridium difficile ciprofloxacin, ofloxacin, norfloxacin Bifidobacterium Lactobacillus Lactobacillus Bacteroides fragilis B. fragilis C. difficile
 Clostridium difficile ciprofloxacin, ofloxacin, norfloxacin Bifidobacterium Lactobacillus Lactobacillus Bacteroides fragilis B. fragilis C. difficile Key words: temafloxacin, TA-167, Bacteroides fragilis,
Clostridium difficile ciprofloxacin, ofloxacin, norfloxacin Bifidobacterium Lactobacillus Lactobacillus Bacteroides fragilis B. fragilis C. difficile Key words: temafloxacin, TA-167, Bacteroides fragilis,
VOL.30 S-1 CHEMOTHERAPY Table 1 Antibacterial activity of CTT against standard strains Table 2 Antibacterial activity of CTT against standard strains
 CHEMOTHERAPY APR. 1982 Fig. 1 Chemical structure of cefotetan (CTT, YM09330) VOL.30 S-1 CHEMOTHERAPY Table 1 Antibacterial activity of CTT against standard strains Table 2 Antibacterial activity of CTT
CHEMOTHERAPY APR. 1982 Fig. 1 Chemical structure of cefotetan (CTT, YM09330) VOL.30 S-1 CHEMOTHERAPY Table 1 Antibacterial activity of CTT against standard strains Table 2 Antibacterial activity of CTT
 Fig. 1. Structures of NM394, NAD-358 and NAD-245 Fig. 2. Typical HPLC chromatograms of NM394 in human plasma by organic solvent extraction method (a): Blank plasma (b): Plasma spiked with NM394 and internal
Fig. 1. Structures of NM394, NAD-358 and NAD-245 Fig. 2. Typical HPLC chromatograms of NM394 in human plasma by organic solvent extraction method (a): Blank plasma (b): Plasma spiked with NM394 and internal
 Osamu NEMOTO, M.D. Clinical and Bacteriological Research of Sucrose/ Povidone-Iodine Ointment (U-PASTA kowa) for Pressure Sores and Skin Ulcers Osamu Nemoto Department of Dermatology,Tonan Hosptal
Osamu NEMOTO, M.D. Clinical and Bacteriological Research of Sucrose/ Povidone-Iodine Ointment (U-PASTA kowa) for Pressure Sores and Skin Ulcers Osamu Nemoto Department of Dermatology,Tonan Hosptal
Feb THE JAPANESE JOURNAL OF ANTIBIOTICS Tebipenem pivoxil 1 1, Meiji Seika 2 Meiji Seika G 3 Meiji Seika Tebipen
 Feb. 2016 THE JAPANESE JOURNAL OF ANTIBIOTICS 69 1 53 53 Tebipenem pivoxil 1 1, 3 2 2 1 1 Meiji Seika 2 Meiji Seika G 3 Meiji Seika 2015 12 15 Tebipenem pivoxil 10% 2010 4 2013 3 3,547 3,540 3,540 3,331
Feb. 2016 THE JAPANESE JOURNAL OF ANTIBIOTICS 69 1 53 53 Tebipenem pivoxil 1 1, 3 2 2 1 1 Meiji Seika 2 Meiji Seika G 3 Meiji Seika 2015 12 15 Tebipenem pivoxil 10% 2010 4 2013 3 3,547 3,540 3,540 3,331
 Table 1. Influence of urine ph on MBCs of new quinolones against Escherichia coli NIHJ JC-2 and Pseudomonas aeruginosa 18S; MBCs in urine were compared with those in Miieller-Hinton broth. Table 2. Influence
Table 1. Influence of urine ph on MBCs of new quinolones against Escherichia coli NIHJ JC-2 and Pseudomonas aeruginosa 18S; MBCs in urine were compared with those in Miieller-Hinton broth. Table 2. Influence
Clinical studies on Cef triaxone in the field of oral surgery JIRO SASAKI,*1 MASATAKA UEMATSU,*l AKIHIRO KANEKO,*1 YOSHIHIDE OHTA,*1 KAZUO SHIIKI,*2 T
 Clinical studies on Cef triaxone in the field of oral surgery JIRO SASAKI,*1 MASATAKA UEMATSU,*l AKIHIRO KANEKO,*1 YOSHIHIDE OHTA,*1 KAZUO SHIIKI,*2 TAKEFUMI MORIHANA,*3 FUMISADA TOMITA,*3 KEN-ICHI MICHI,*4
Clinical studies on Cef triaxone in the field of oral surgery JIRO SASAKI,*1 MASATAKA UEMATSU,*l AKIHIRO KANEKO,*1 YOSHIHIDE OHTA,*1 KAZUO SHIIKI,*2 TAKEFUMI MORIHANA,*3 FUMISADA TOMITA,*3 KEN-ICHI MICHI,*4
Key words: Norfloxacin (NFLX), Infectious enteritis, Double-blind method
 Key words: Norfloxacin (NFLX), Infectious enteritis, Double-blind method Fig. 1 Chemical structure Norfloxacin (NFLX) Pipemidic acid (PPA) Fig. 2 Package of test drugs Table 1 Criteria for evaluation
Key words: Norfloxacin (NFLX), Infectious enteritis, Double-blind method Fig. 1 Chemical structure Norfloxacin (NFLX) Pipemidic acid (PPA) Fig. 2 Package of test drugs Table 1 Criteria for evaluation
 Takeji NISHIKAWA, M. D.*, Takashi HARADA, M. D.** and Tatsuya IZUML M. D. Toshiyuki SUGI, M. D. Hiroshi HANYAKU, M. D. Shun-ichi MIYAKAWA, M. D. and Masaru TANAKA, M. D. Nobuko INAMOTO, M. D. and Hironori
Takeji NISHIKAWA, M. D.*, Takashi HARADA, M. D.** and Tatsuya IZUML M. D. Toshiyuki SUGI, M. D. Hiroshi HANYAKU, M. D. Shun-ichi MIYAKAWA, M. D. and Masaru TANAKA, M. D. Nobuko INAMOTO, M. D. and Hironori
 Key words : R-plasmid, Urinary tract infection, E. coli Fig. 1. MIC distribution against E. coli isolated from urinary tract (366 strains) and isolation - frequencies of drug-resistant strains Table 1.
Key words : R-plasmid, Urinary tract infection, E. coli Fig. 1. MIC distribution against E. coli isolated from urinary tract (366 strains) and isolation - frequencies of drug-resistant strains Table 1.
VOL.39 S-3
 VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
 CHEMOTHERAPY AUG. 1982 VOL. 30 NO. 8 CHEMOTHERAPY Fig.1 Relation between various-closis of cefazolin and detection rate of organisms in heart blood of dying mice with E. coli and P. aeruginosa infection
CHEMOTHERAPY AUG. 1982 VOL. 30 NO. 8 CHEMOTHERAPY Fig.1 Relation between various-closis of cefazolin and detection rate of organisms in heart blood of dying mice with E. coli and P. aeruginosa infection
VOL. 23 NO. 3 CHEMOTHERAPY 1379 Table 1 Susceptibility of clinical isolated strains to Tobramycin
 VOL. 23 NO. 3 CHEMOTHERAPY 1379 Table 1 Susceptibility of clinical isolated strains to Tobramycin 1380 CHEMOTHERAPY MAR. 1975 Table 2 Susceptibility of isolated Pseudomonas aeruginosa to various antibiotics
VOL. 23 NO. 3 CHEMOTHERAPY 1379 Table 1 Susceptibility of clinical isolated strains to Tobramycin 1380 CHEMOTHERAPY MAR. 1975 Table 2 Susceptibility of isolated Pseudomonas aeruginosa to various antibiotics
CHEMOTHERAPY DEC Table 1 Antibacterial spectra of T-1982, CTT, CMZ, CTX, CPZ and CEZ 106 CFU/ml Note: P; Peptococcus, S; Streptococcus, G; Gaffk
 VOL. 30 S-3 CHEMOTHERAPY imeumoniae, Serratia marcescens, Proteus sp, CHEMOTHERAPY DEC. 1982 Table 1 Antibacterial spectra of T-1982, CTT, CMZ, CTX, CPZ and CEZ 106 CFU/ml Note: P; Peptococcus, S; Streptococcus,
VOL. 30 S-3 CHEMOTHERAPY imeumoniae, Serratia marcescens, Proteus sp, CHEMOTHERAPY DEC. 1982 Table 1 Antibacterial spectra of T-1982, CTT, CMZ, CTX, CPZ and CEZ 106 CFU/ml Note: P; Peptococcus, S; Streptococcus,
日本化学療法学会雑誌第65巻第4号
 Mycoplasma pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae Key words Mycoplasma pneumoniae Mycoplasma pneumoniae M. pneumoniae I M. pneumoniae M. pneumoniae M. pneumoniae
Mycoplasma pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae Key words Mycoplasma pneumoniae Mycoplasma pneumoniae M. pneumoniae I M. pneumoniae M. pneumoniae M. pneumoniae
 Table 1 Survival rates of infected mice given antibiotic doses producing peak serum a) S. aurcus Smith Challenge dose :7 ~10 (5% mucin) CFU/mouse. LD50: 1 ~103 (5% mucin) CFU/mouse. Table 2 Survival rates
Table 1 Survival rates of infected mice given antibiotic doses producing peak serum a) S. aurcus Smith Challenge dose :7 ~10 (5% mucin) CFU/mouse. LD50: 1 ~103 (5% mucin) CFU/mouse. Table 2 Survival rates
Hisao Takayasu Department of Urology, Faculty of Medicine, University of Tokyo Masaaki Ohkoshi Department of Urology, Tokai University School of Medic
 COMPARATIVE CLINICAL EFFECT OF AMIKACIN AND GENTAMICIN ON COMPLICATED URINARY TRACT INFECTIONS BY DOUBLE-BLIND METHOD Tsuneo Nishiura and Yukimichi Kawada Department of Urology, Gifu University School
COMPARATIVE CLINICAL EFFECT OF AMIKACIN AND GENTAMICIN ON COMPLICATED URINARY TRACT INFECTIONS BY DOUBLE-BLIND METHOD Tsuneo Nishiura and Yukimichi Kawada Department of Urology, Gifu University School
日本化学療法学会雑誌第60巻第4号
 Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Key words β Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae μ μ S. pneumoniae H. influenzae S. pneumoniae
Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Key words β Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae μ μ S. pneumoniae H. influenzae S. pneumoniae
 Table 1. Antibacterial activity of cefdinir, cefixime, cefteram, cefuroxime, cefaclor and amoxicillin against standard strains Inoculum size: 108 cells/ml CFDN: cefdinir, CFIX: cefixime, CFTM: cefteram,
Table 1. Antibacterial activity of cefdinir, cefixime, cefteram, cefuroxime, cefaclor and amoxicillin against standard strains Inoculum size: 108 cells/ml CFDN: cefdinir, CFIX: cefixime, CFTM: cefteram,
Key words: E. coli O 157: H7, fosfomycin, verotoxin, mouse infection
 Key words: E. coli O 157: H7, fosfomycin, verotoxin, mouse infection Table 1. Bacterial cell counts in feces of mice infected with Esclwrichia coli O 157: H7 NK2 before and during oral dosing with fosfomycin
Key words: E. coli O 157: H7, fosfomycin, verotoxin, mouse infection Table 1. Bacterial cell counts in feces of mice infected with Esclwrichia coli O 157: H7 NK2 before and during oral dosing with fosfomycin
 CHEMO THER APY Table 1. Background of the volunteers in the phase I clinical trial of ME1207 Table 2. Methods for Phase I clinical trial of ME1207 All doses are expressed in terms of potency as ME1206
CHEMO THER APY Table 1. Background of the volunteers in the phase I clinical trial of ME1207 Table 2. Methods for Phase I clinical trial of ME1207 All doses are expressed in terms of potency as ME1206
VOL.48 NO.7 lase negative staphylococci, Escherichia coli, Klebsiella spp., Citrobacter freundii, Enterobacter spp., indole-positive Proteus, Serratia
 ceftazidime, cefpirome, cefepime, cefoperazone/sulbactam (2: 1), imipenem Staphylococcus aureus oxacillin coagulase negative staphylococci Escherichia coli piperacillin Klebsiellac spp. Citrobacter Pseudomonas
ceftazidime, cefpirome, cefepime, cefoperazone/sulbactam (2: 1), imipenem Staphylococcus aureus oxacillin coagulase negative staphylococci Escherichia coli piperacillin Klebsiellac spp. Citrobacter Pseudomonas
日本化学療法学会雑誌第56巻第S-1号
 Key words Streptococcus pneumoniae µ µ H F Cl H H N N H N F COOH O Fig.1. Sitafloxacinstructure. I γ S. pneumoniae Haemophilus influenzae Table1. Observationandtestschedule Observation/Tests Informedconsent
Key words Streptococcus pneumoniae µ µ H F Cl H H N N H N F COOH O Fig.1. Sitafloxacinstructure. I γ S. pneumoniae Haemophilus influenzae Table1. Observationandtestschedule Observation/Tests Informedconsent
CHEMOTHERAPY APR Fig. 2 The inactivation of aminoglycoside antibiotics by PC-904 Fig. 3 Serum concentration of PC-904 (1) Fig. 4 Urinary recover
 VOL.26 S-2 CHEMOTHERAPY Gentamicin (GM), Dibekacin (DKB), Tobramycin Fig. 1 Protein concentration and protein binding rate Table 2 Protein binding rate of PC-904 in serum of healthy adults, and patients
VOL.26 S-2 CHEMOTHERAPY Gentamicin (GM), Dibekacin (DKB), Tobramycin Fig. 1 Protein concentration and protein binding rate Table 2 Protein binding rate of PC-904 in serum of healthy adults, and patients
208 ( 2 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 63 _ 3 June 2010 Cefditoren pivoxil (CDTR-PI) MS MS 10%
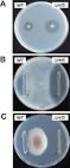 207 ( 1 ) Cefditoren pivoxil G 2010 2 2 2006 3 Cefditoren pivoxil CDTR-PI MS 10% CDTR-PI 305 2,144 2,006 1,958 1.79% 36 2,006 26 (1.30%) CDTR-PI CDTR-PI (9 mg/kg/day) 1.5 2 (2.70%) 2 (1.92%) 93.5% 1,831
207 ( 1 ) Cefditoren pivoxil G 2010 2 2 2006 3 Cefditoren pivoxil CDTR-PI MS 10% CDTR-PI 305 2,144 2,006 1,958 1.79% 36 2,006 26 (1.30%) CDTR-PI CDTR-PI (9 mg/kg/day) 1.5 2 (2.70%) 2 (1.92%) 93.5% 1,831
CHEMOTHERAPY OCT Fig. 1 Chemical structure of CVA-K
 OCT. 1986 Fig. 1 Chemical structure of CVA-K VOL.34 S-4 heart infusion broth (Difco) 37.0 g, Resazurin 0.1% alcoholic solution (Wako) 1.0 ml, L-Cystein-HCl H2O (Wako) 0.5 g, Bact agar (Difco) 1.0 g, Deionized
OCT. 1986 Fig. 1 Chemical structure of CVA-K VOL.34 S-4 heart infusion broth (Difco) 37.0 g, Resazurin 0.1% alcoholic solution (Wako) 1.0 ml, L-Cystein-HCl H2O (Wako) 0.5 g, Bact agar (Difco) 1.0 g, Deionized
CHEMOTHERAPY JUNE 1986
 VOL. 34 S-3 CHEMOTHERAPY Fig. 1 Structural formula of L-105 CHEMOTHERAPY JUNE 1986 VOL. 34 S-3 CHEMOTHERAPY Table 1 Antibacterial spectra of L-105 against gram negative anaerobic rods Inoculum 106 cells/ml
VOL. 34 S-3 CHEMOTHERAPY Fig. 1 Structural formula of L-105 CHEMOTHERAPY JUNE 1986 VOL. 34 S-3 CHEMOTHERAPY Table 1 Antibacterial spectra of L-105 against gram negative anaerobic rods Inoculum 106 cells/ml
CHEMOTHERAPY JUNE 1987 Table1 Media used *BHIB, brain heart infusion broth (Difco); /3 -NAD, S -nicotinamidoadeninedinucleotide (Sigma Chemical Co.);
 VOL.35 S-2 CHEMOTHERAPY Fig.1 Chemical structure of carumonam CHEMOTHERAPY JUNE 1987 Table1 Media used *BHIB, brain heart infusion broth (Difco); /3 -NAD, S -nicotinamidoadeninedinucleotide (Sigma Chemical
VOL.35 S-2 CHEMOTHERAPY Fig.1 Chemical structure of carumonam CHEMOTHERAPY JUNE 1987 Table1 Media used *BHIB, brain heart infusion broth (Difco); /3 -NAD, S -nicotinamidoadeninedinucleotide (Sigma Chemical
 Table 1 Classification of female patients with vealcal irritating symptom by their signs Urination pain with other vesical irritability or not Table 2 Serum levels of DL-8280 after a single oral administration
Table 1 Classification of female patients with vealcal irritating symptom by their signs Urination pain with other vesical irritability or not Table 2 Serum levels of DL-8280 after a single oral administration
Key words : candidemia, endotoxin, D-arabinitol, Candida antigen, serological examination
 Key words : candidemia, endotoxin, D-arabinitol, Candida antigen, serological examination Fig. 1 The value of fungal index and D- arabinitol/creatinine. Fungal index was mesured by Endotoxin D and Endospecy
Key words : candidemia, endotoxin, D-arabinitol, Candida antigen, serological examination Fig. 1 The value of fungal index and D- arabinitol/creatinine. Fungal index was mesured by Endotoxin D and Endospecy
co Kanamycin (KM), Streptomycin (SM), Fradiomycin Table 2. Comparison of antibacterial activity of 3', 4'-dideoxykanamycin B with that of other antibi
 Fig. 1. Chemical structure of 3', 4'-dideoxykanamycin B co Kanamycin (KM), Streptomycin (SM), Fradiomycin Table 2. Comparison of antibacterial activity of 3', 4'-dideoxykanamycin B with that of other antibiotics
Fig. 1. Chemical structure of 3', 4'-dideoxykanamycin B co Kanamycin (KM), Streptomycin (SM), Fradiomycin Table 2. Comparison of antibacterial activity of 3', 4'-dideoxykanamycin B with that of other antibiotics
 VOL.32 S-9 CHEMOTHERAPY Table 1 Minimum inhibitory concentrations of AC-1370, CPZ and CAZ Table 2 Efficacy of AC-1370 and CPZ against systemic infections in mice *Inoculum size: 106 cells/ml * 95% confidence
VOL.32 S-9 CHEMOTHERAPY Table 1 Minimum inhibitory concentrations of AC-1370, CPZ and CAZ Table 2 Efficacy of AC-1370 and CPZ against systemic infections in mice *Inoculum size: 106 cells/ml * 95% confidence
CHEMOTHERAPY
 VOL.40 S-1 coagulase negative Staphylococcus sp, methicillin- resistant Staphylococcus aureus (MRSA), Enterococcus faecalis, Escherichia coli, Citrobacter freundii, Klebsiella pneumoniae, Enterobacter
VOL.40 S-1 coagulase negative Staphylococcus sp, methicillin- resistant Staphylococcus aureus (MRSA), Enterococcus faecalis, Escherichia coli, Citrobacter freundii, Klebsiella pneumoniae, Enterobacter
CHEMOTHERAPY SEPT. 1991
 CHEMOTHERAPY SEPT. 1991 VOL. 39 S-3 Table 1. Background of characteristics and allocation of 9 healthy male volunteers in a single-dose study on betamipron Table 2. Background of characteristics and allocation
CHEMOTHERAPY SEPT. 1991 VOL. 39 S-3 Table 1. Background of characteristics and allocation of 9 healthy male volunteers in a single-dose study on betamipron Table 2. Background of characteristics and allocation
2108 CHEMOTHERAPY SEPT Table 1 Antimicrobial spectrum Fig. 1
 2108 CHEMOTHERAPY SEPT. 1977 Table 1 Antimicrobial spectrum Fig. 1 VOL. 25 NO. 7 CHEM 014 HERAPY 2109 Table 2 Susceptibility distribution of Staphylococcus aureus to aminoglycosides (54 strains) Table
2108 CHEMOTHERAPY SEPT. 1977 Table 1 Antimicrobial spectrum Fig. 1 VOL. 25 NO. 7 CHEM 014 HERAPY 2109 Table 2 Susceptibility distribution of Staphylococcus aureus to aminoglycosides (54 strains) Table
Fig. 1 Trends of TB incidence rates for all forms and smear-positive pulmonary TB in Kawasaki City and Japan. Incidence=newly notified cases of all fo
 Kekkaku Vol. 79, No. 1: 17-24, 2004 17 (Received 21 Aug. 2003/Accepted 18 Nov. 2003) Fig. 1 Trends of TB incidence rates for all forms and smear-positive pulmonary TB in Kawasaki City and Japan. Incidence=newly
Kekkaku Vol. 79, No. 1: 17-24, 2004 17 (Received 21 Aug. 2003/Accepted 18 Nov. 2003) Fig. 1 Trends of TB incidence rates for all forms and smear-positive pulmonary TB in Kawasaki City and Japan. Incidence=newly
