untitled
|
|
|
- しほこ ひらみね
- 5 years ago
- Views:
Transcription
1
2 mg 0.035mg 2 1
3
4 NET 1.0mg EE 0.035mg *NET *NET *NET *NET 88 NSAIDs GnRH GnRH NSAIDs
5 2 1 2 PVC PTP PET PE *NET *NET *NET 1 PTP PVC / 4075 RH 6 PVC PTP 4075 RH 6 4
6 PTP PVC PTP PET PE 1 2TLC RH / 4075 RH 6 / 50 / RH/ 3 D lx lx hr W hr/m 2 / 8 TLC
7 / ~
8 *NET *NET NET P 2.45 EE17β- E NET EE in vivo P E NET EE 1000 NET 20mg/kg EE 1mg/kg NET EE ALT AST 4 *NET E2P 1 MMP 7
9 in vitro E2 P MMP-3 MMP-7 J Clin Endocrinol Metab 87(10): , athymic ncr/nude E E2 P Fertil Steril 83:529-37, EE LPSLPS EE ALT AST Alcohol Clin Exp Res 26(8):70S-74S,2002 *NET *NET *NET C NET 14 C-NET 3mg/kg0.15mg/kg 1 Cmax C EE 14 C-EE 0.15mg/kg+NET 3mg/kg 0.5 Cmax BA NET 16 54EE 4 14 C-NET 3mg/kg 0.15mg/kg 14 C-EE 0.15mg/kg+NET 3mg/kg
10 14 C-NET 3mg/kg 0.15mg/kg 14 C-EE 0.15mg/kg+NET 3mg/kg NET EE C-NET 3mg/kg 0.15mg/kg 14 C-EE 0.15mg/kg+NET 3mg/kg 30 NET 96EE NET EE C-NET 3mg/kg 0.15mg/kg 30 8 NET NET 14 C-EE 0.15mg/kg+NET 3mg/kg EE 8 EE 5 in vitro C-NET 3mg/kg EE 0.15mg/kg C-EE 0.15mg/kg+NET 3mg/kg NET EE H-EE NET EE 2 *NET 9
11 1 EE mg/kg EE Cmax 6 J Chromatogr 793:309-15, α NET EE EE Drug Metab Disp 29:76-71&976-82, EE 1mg/kg EE AUC Cmax Contraception 62:253-57,2000., Contraception 70:423-27,2004 *NET 4 1 *NET NET LC/MS/MSRIA100 30pg/mLEE LC/MS/MS RIA ELISA pg/mL IKH-01-3 *NET BE *NET 1 10
12 mL 1 Cmax AUCt AUC *NET 90 NET log 0.856log log 0.925log 1.030EE log 0.898log log log log 0.80log IKH * 三相性 * 三相性 * 三相性 * 三相性 mg /0.750mg/1.000mg 0.035mg E2 pg/ml 41.9± ± ±* 三相性 U/mL 36.5± ±0.2 * 三相性 83.6± ±0.2 CA ± ± IKH CA125 U/mL 26.62± ± ± IKH CA125 U/mL 21.57± ± ± ±0.30 ± 5 in vitro NET A 3α 3β-hydroxy EE 2 2-hydroxy 2 3-metylether EE UGT1A1 1A4 1A8 UGT Ann Rev Pharmacol Toxicol 40: , *NET NET 7 *NET NET NET NET NET NET NET T max 1.1±0.2 Cmax 10.8±2.6ng/mL AUC ±14.0ng h/ml 11
13 t 1/2 6.3±1.0 EE 1.1± ±35.4pg/mL 1266±324pg h/ml 12.0±4.7 9 *NET NET T max 2.6±3.7 Cmax 22.4±11.1ng/mL AUC ±51.3ng h/ml t 1/2 9.7±2.2 EE 1.3± ±52pg/mL 1999±455pg h/ml 12.5±2.7 RIA NET 0.5mg 0.035mg 21 FSH LH NET EE DSG 0.15mg 0.030mg 21 DSG 0.050mg 0.125mg 15 FSH LH DSG 0.150mg 0.030mg 21 EE 0.050mg 7 DSG 0.150mg 0.050mg 15 E2 E2 E PG 0.150mg 0.030mg PGF 2α NET PG r-afs in vitro E mol/l 10-6 mol/l TNF α IL -8 12
14 5 EE E2 E2 EE NET 1 *NET *NET BE 1 NET 1.0mg EE 0.035mg BE BE *NET *NET BE EE NET ph1.2 ph ph1.2 ph ph1.2 ph *NET 1 NET LC/MS/MS EE ELISA LC/MS/MS EE ELISA LC/MS/MS RIA ELISA 17β
15 0.2NET ELISA NET 2 3 NET EE T max 1.5 t 1/2 7 9 NET NET ELISA ELISA NET 3α-hydroxy-5α-steroid NET BE BE BA *NET BE ph1.2 ph NET *NET BE BE BE BE *NET 2 NSAIDs GnRH NSAIDs 5 NSAIDs 14
16 *NET *NET NET EE GnRH 3 EE EE NET NET EE EE 15
17 IKH Andersch Fertil Steril. 60(1):75-9,1993 Biberoglu Fertil Steril. 77(1):52-61, * 三相性 NET mg/0.750mg/1.000mg 0.035mg * 三相性 17 Full Analysis Set FAS NET NET * 三相性 17 Per Protocol Set PPS 16
18 ± 4.5± ±1.6 * 三相性 NET 4.5± ±1.7 p< t -2.3±1.4 * 三相性 NET -2.5±1.5 p= t * 三相性 NET * 三相性 * 三相性 NET NET n n n n / * 三相性 NET 14/ * 三相性 NET 17
19 IKH ± FAS CA125 IL-6-2.0± ±1.42 p< t
20 3 FAS -1 ) t 1) p< t 1) P= FAS t 1) p< ) 5 5 FAS N ± VAS 1, ± ±1.46 1, ± ± ± ±22.87 VAS 1, ± ± N ,3 mm CA125 2, U/mL p< ) p= ) p= ) p= ) p= ) 19
21 IL-6 2, ) pg/ml t 62 Wilcoxon p= ) 5 p= p= Wilcoxon 49/ / n n n n n 40/ / / INR IKH
22 FAS PPS FAS 1 t 1) p= p< p< p< p< p< p< p< p< p< p= p< p< t /
23 114/ / PT B B () 10 B03-07 ( ) 13 () 13 B06-03 ( ) 89 () 180 B10-01 ()( ) 5 B B () 1 B16-04 () 1 ( ) 1 B16-08 () 5 B17-01 () 86 B17-05 AST ALT γ-gtp () 172 B18-07 ALT AST γ-gtp Al-P () 88 B20-08 () 89 B B21-06 () n n n 22
24 IKH *NET *NET *NET 10 9 *NET 31 13/ *NET *NET 17/ *NET *NET 1 1 NSAIDs NSAIDs NSAIDs GnRH NSAIDs 23
25 GnRH GnRH GnRH 24
26 2 1 Verbal Rating Scale VRS Andersch Am J Obstet Gynecol. 144(6):655-60,1982Biberoglu Am J Obstet Gynecol. 139(6):645-54,1981 Am J Obstet Gynecol. 175: ,1996 Andersch 3 Biberoglu Dysmenorrhea Nonmenstrual pain Deep dyspareunia 4 10 Andersch Dysmenorrhea and nonmenstrual pelvic pain Symptom and score Sum of the three groups Limitation of ability to work Coexistence of systemic Need for analgesics Mild : : Unaffected symptoms 0 : No Moderate : 4 and 5 1 : Rarely affected 0 : Absent 1 : Rarely Severe : 6 and 7 2 : Moderately affected 1 : Present 2 : Regularly 3 : Clearly inhibited 3 : Inefficacious Symptom and score Dysmenorrhea Nonmenstrual pain Deep dyspareunia 11 Biberoglu Severity 0 : Absence of pain 1 : Some loss of work efficiency Mild 2 : In bed part of 1 day, Occasional loss of work Moderate 3 : In bed for 1 or more days, Incapacitation Severe 0 : Absence of pain 1 : Occasional pelvic discomfort Mild 2 : Noticeable discomfort for most of the cycle Moderate 3 : Pain persisting during the cycle or requiring strong analgesics Severe 0 : No discomfort 1 : Tolerated discomfort Mild 2 : Intercourse painful to the point of interruption Moderate 3 : Intercourse avoided because of pain Severe 25
27 12 Biberoglu Category Score Description Dysmenorrhea 0 Absent No discomfort 1 Mild Some loss of work efficiency. Use of mild analgesics 2 Moderate Occasional loss of work efficiency. Use of moderate analgesics 3 Severe Incapacitation. Use of strong analgesics VAS VRS Andersch Biberoglu Andersch Andersch Biberoglu VRS VRS Andersch 26
28 13 VAS 13 FAS ) p= p= p< p= p= p= p< ) p= p= p= p= p= p= p=
29 2 1/2 1/2 2/3 2/3-1 ±49 7.0± ± ± ± /2 1/2 2/3 2/3 3/4 3/4 * 三相性 NET Fertil and Steril. 60(1):75-9, GnRH 4 28
30 ± ±1.42 p< t VAS CA125 CA
31 123 67/ / Beecham 30
32 American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Number 310, April Endometriosis in adolescents. Obstet Gynecol. 105(4):921-7,2005 NSAID GnRH OC 31
33 3 2 3 CA
34 Royal College of Obstetricians and Gynecologists, 2000ACOG Committee on Practice Bulletins-Gynecology, Clinical management guidelines for obstetrician-gynecologists.1999 Novak's Gynecology. 13th edition. p951-2,2002 Fertil Steril. 78:961-72,2002 N Engl J Med. 345:266-75,2001 *NET GnRH Fertil Steril. 60(1):75-9,1993 Fertil Steril. 77(1):52-61, *NET 1 NET1.0mgEE0.035mg * 三相性 NET
35 GnRH GnRH 34
36 CTD /
37 *NET *NET
38 OC WHO ± ± mm ± mm 3 p= t 37
39 ± mm ± mm 3 p= t 29.1% 48/ /30 p= Fisher % 11/ /30 p= Fisher
40
41 GnRH 2 40
42 3 4 1 *NET *NET 2 GnRH 4 41
43 5 18 GnRH
44
45 DSG 0.050mg 0.125mg EE0.05mg 7 EE0.05mg DSG0.125mg DSG 0.150mg DSG 0.125mg / B03-0 B
46 GCP GCP
2.7.6 MJR a MRI CT b 2 Beecham r-afs mg/ mg/ Gn-RH 742
 MJR-35 34 1 1 a MRICT b 2Beecham r-afs 3 4 20 4 mg/ 20 40 5 6 2 1 2 3 44 mg/ 5 6 7 8 4 Gn-RH 742 9 10 11 12 1 20 60 1 1MJR-351 mg/ 0.25 2 Lot. No. 2MJR-352 mg/ 1 1 Lot. No. 3MJR-354 mg/ 1 2 Lot. No. 2
MJR-35 34 1 1 a MRICT b 2Beecham r-afs 3 4 20 4 mg/ 20 40 5 6 2 1 2 3 44 mg/ 5 6 7 8 4 Gn-RH 742 9 10 11 12 1 20 60 1 1MJR-351 mg/ 0.25 2 Lot. No. 2MJR-352 mg/ 1 1 Lot. No. 3MJR-354 mg/ 1 2 Lot. No. 2
 200mg 2005 3 876179 2002 3 3 200mg VFEND for Intravenous Use (1) (2) [4.1 ] (3) [4.2 ] (1) [1] (2) (3) [1] 3
200mg 2005 3 876179 2002 3 3 200mg VFEND for Intravenous Use (1) (2) [4.1 ] (3) [4.2 ] (1) [1] (2) (3) [1] 3
 CTD 2 2.6 5.3mg 0.4mg 0.6mg 0.8mg 1.0mg 1.2mg 1.4mg 1.6mg 1.8mg 2.0mg 12mg 2.6 Page 3 2.6.6...5 2.6.6.1...6 2.6.6.2...9 2.6.6.3...9 2.6.6.4... 11 2.6.6.5... 11 2.6.6.6... 11 2.6.6.7... 11 2.6.6.8... 11
CTD 2 2.6 5.3mg 0.4mg 0.6mg 0.8mg 1.0mg 1.2mg 1.4mg 1.6mg 1.8mg 2.0mg 12mg 2.6 Page 3 2.6.6...5 2.6.6.1...6 2.6.6.2...9 2.6.6.3...9 2.6.6.4... 11 2.6.6.5... 11 2.6.6.6... 11 2.6.6.7... 11 2.6.6.8... 11
(1) ) ) (2) (3) (4) (5) (1) (2) b (3)..
 ... -1... -1... -2... -6.... -1 (1)... -1 1)... -1 2)... -14 (2)... -19 (3)... -52 (4)... -18 (5)... -136.... -196 (1)... -196 (2) b... -224 (3)... -233.... -251.... -286.... -289 (1)... -289 (2)... -32
... -1... -1... -2... -6.... -1 (1)... -1 1)... -1 2)... -14 (2)... -19 (3)... -52 (4)... -18 (5)... -136.... -196 (1)... -196 (2) b... -224 (3)... -233.... -251.... -286.... -289 (1)... -289 (2)... -32
C033** of 7 C034** PEG-IFN-2b + IFN -2b + 78 HCV-RNA PEG-IFN-2b IFN-2b n=250 n=200 n= =10 % % 85 % 74 % %
 2.7.6.9 2.7.6.9.1 Genotype 1 C PEG-IFN-2b+ C033* Genotype 1 C PEG-IFN-2b + IFN-2b + 2.7.6-54 2.7.6-54 C033* 1 of 7 Genotype 1 C PEG-IFN-2b+ Genotype 1 C PEG-IFN-2b + IFN-2b + 24 HCV-RNA 48 24 follow-up
2.7.6.9 2.7.6.9.1 Genotype 1 C PEG-IFN-2b+ C033* Genotype 1 C PEG-IFN-2b + IFN-2b + 2.7.6-54 2.7.6-54 C033* 1 of 7 Genotype 1 C PEG-IFN-2b+ Genotype 1 C PEG-IFN-2b + IFN-2b + 24 HCV-RNA 48 24 follow-up
untitled
 19 11 13 300 18 3 31 1300mg 4 6 C 15 H 22 FN 3 O 6 359.35 + -1-5--β-D--5--1,2--2- -4- 19 11 13 300 18 3 31 A B B A 30 1 2 21 7 1 1 1.31m 2 900mg 1.31m 2 1.64m 2 1,200mg 1.64m 2 1,500mg B 30 1 2 14 7 1
19 11 13 300 18 3 31 1300mg 4 6 C 15 H 22 FN 3 O 6 359.35 + -1-5--β-D--5--1,2--2- -4- 19 11 13 300 18 3 31 A B B A 30 1 2 21 7 1 1 1.31m 2 900mg 1.31m 2 1.64m 2 1,200mg 1.64m 2 1,500mg B 30 1 2 14 7 1
125 2 P 1st washout 2 PB P mg/dL nd washout 2 P 5.5mg/dL< mg/dL <2.5mg/dL P P 2 D D 3 Ca 10
 . (1) 125 1 125 Renagel PB-94 P intact-pth P 1 b c a b 1 18 2 3 3 3 1 P 4 D 1 5 6 7 2 1HIV 2 3 4 5Hb 8.0g/dL ALT 48IU/L 6 7 PB-94 440mg 403mg 1-196 125 2 P 1st washout 2 PB-94 1 2 4 4 P 2.5 5.5mg/dL 1
. (1) 125 1 125 Renagel PB-94 P intact-pth P 1 b c a b 1 18 2 3 3 3 1 P 4 D 1 5 6 7 2 1HIV 2 3 4 5Hb 8.0g/dL ALT 48IU/L 6 7 PB-94 440mg 403mg 1-196 125 2 P 1st washout 2 PB-94 1 2 4 4 P 2.5 5.5mg/dL 1
untitled
 COLAK Mehmed 11000 305 305 180 10000 9000 8000 160 140 120 7000 100 6000 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 80 GH IGF-1 GH GH 5 4 3 2 1 0 2 3 4 5
COLAK Mehmed 11000 305 305 180 10000 9000 8000 160 140 120 7000 100 6000 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 80 GH IGF-1 GH GH 5 4 3 2 1 0 2 3 4 5
1.8.2 Page MIC () MIC 50 / MIC 90 µg/ml Candida albicans (54) / Candida glabrata (25) 0.25 / 0.5 Candida guilliermondii a) (2)
 1.8.2 Page1 1.8.2 96% 2002 40 B 1 123 (1) in vitro 1) in vitro C. glabrata C. krusei 1 2 19951997 3 1999 2002 MIC MIC 2 1.8.2 Page2 1 19951997 MIC () MIC 50 / MIC 90 µg/ml Candida albicans (54) 0.016 /
1.8.2 Page1 1.8.2 96% 2002 40 B 1 123 (1) in vitro 1) in vitro C. glabrata C. krusei 1 2 19951997 3 1999 2002 MIC MIC 2 1.8.2 Page2 1 19951997 MIC () MIC 50 / MIC 90 µg/ml Candida albicans (54) 0.016 /
ケイセントラ_製品情報概要_H1-4_収載_新発売
 DIC 135-627 12 CONTENTS 34 32 33 1 2 K 34 3 4 5 12 12 18 24 3 31 31 31 33 KVKA K K VKA VKA K - PT-INR KC S 1996VKA VKA 3 KK CS 33 34 35 35 35 36 36 K 32 33 213 4VKA 12VKAFDA Kcentra 42 217 1 in vitro 1
DIC 135-627 12 CONTENTS 34 32 33 1 2 K 34 3 4 5 12 12 18 24 3 31 31 31 33 KVKA K K VKA VKA K - PT-INR KC S 1996VKA VKA 3 KK CS 33 34 35 35 35 36 36 K 32 33 213 4VKA 12VKAFDA Kcentra 42 217 1 in vitro 1
2007050781......_.L.O...z.W
 a s d f g a s d f g h j k l 0 in vitro in vivo in vitro in vivo in vivo in vivo in vitro Metabolism Metabolism Endocr Endocr Endocrinol Ann NYA cad Sci J clin Endocr Metab
a s d f g a s d f g h j k l 0 in vitro in vivo in vitro in vivo in vivo in vivo in vitro Metabolism Metabolism Endocr Endocr Endocrinol Ann NYA cad Sci J clin Endocr Metab
終末期癌患者に対する 輸液治療の是非
 3 A B NSAID D Case stories A B NSAID D Case stories A. IASP Task Force on Taxonomy WHO Collaborating Center for Palliative Cancer Care : Looking forward to cancer pain relief for all. CBC Oxford, Oxford,
3 A B NSAID D Case stories A B NSAID D Case stories A. IASP Task Force on Taxonomy WHO Collaborating Center for Palliative Cancer Care : Looking forward to cancer pain relief for all. CBC Oxford, Oxford,
=PAS.. =.. =.... 1% 01% ] = = = = 4 (A/A 2 / /B ) 1 8 2n / n n 64 n 64 n ] ] 4
![=PAS.. =.. =.... 1% 01% ] = = = = 4 (A/A 2 / /B ) 1 8 2n / n n 64 n 64 n ] ] 4 =PAS.. =.. =.... 1% 01% ] = = = = 4 (A/A 2 / /B ) 1 8 2n / n n 64 n 64 n ] ] 4](/thumbs/91/107033392.jpg) 1 1 1 1.1 1.1.1 3 : 0: + : +0 : : } 40 40 40 = 3 35 35 (38 )? +0+7 30 35-0+0 38 38 +30 5 30 30 +20 16 16 1. [ ] 2. 3. 4. + = etc 1.1.2 Ru 1 2 1.2 1.2.1 =PAS.. =.. =.... 1% 01% ] 1.2.2 = = = = 4 (A/A 2
1 1 1 1.1 1.1.1 3 : 0: + : +0 : : } 40 40 40 = 3 35 35 (38 )? +0+7 30 35-0+0 38 38 +30 5 30 30 +20 16 16 1. [ ] 2. 3. 4. + = etc 1.1.2 Ru 1 2 1.2 1.2.1 =PAS.. =.. =.... 1% 01% ] 1.2.2 = = = = 4 (A/A 2
sick contact1l
 Clinical Question 2017 年 5 月 15 日 分野 : 内分泌テーマ : 治療 19 14 15 18 sick contact1l 170cm/68.8kg, GCSE1V1M5, HR: 120/min, BP: 104/60mmHg, RR: 30/min, SpO 2 : 96%, BT: 38.0 WBC Hb PLT CRP Cr BUN 23400 15.0 35.5
Clinical Question 2017 年 5 月 15 日 分野 : 内分泌テーマ : 治療 19 14 15 18 sick contact1l 170cm/68.8kg, GCSE1V1M5, HR: 120/min, BP: 104/60mmHg, RR: 30/min, SpO 2 : 96%, BT: 38.0 WBC Hb PLT CRP Cr BUN 23400 15.0 35.5
HOE901 A S S Gly Ile Val Glu Gln Cys Cys Thr Ser Ile Cys Ser Leu Tyr Gln Leu Glu Asn Tyr Cys Gly
 HOE901 A S S Gly Ile Val Glu Gln Cys Cys Thr Ser Ile Cys Ser Leu Tyr Gln Leu Glu Asn Tyr Cys Gly 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 S S S Phe Val Asn Gln His Leu Cys Gly Ser His Leu
HOE901 A S S Gly Ile Val Glu Gln Cys Cys Thr Ser Ile Cys Ser Leu Tyr Gln Leu Glu Asn Tyr Cys Gly 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 S S S Phe Val Asn Gln His Leu Cys Gly Ser His Leu
エディロールカプセルインタビューフォーム
 2012 4 5 873112 2008 () 1 0.5g 0.5g 0.75g 0.75g JAN EldecalcitolJAN 2011 1 21 2011 3 11 2011 4 11 TEL0120-591-818 03-3985-5599 8:3017:00 http://www.taishotoyama.co.jp/ 20124 http://www.info.pmda.go.jp/
2012 4 5 873112 2008 () 1 0.5g 0.5g 0.75g 0.75g JAN EldecalcitolJAN 2011 1 21 2011 3 11 2011 4 11 TEL0120-591-818 03-3985-5599 8:3017:00 http://www.taishotoyama.co.jp/ 20124 http://www.info.pmda.go.jp/
untitled
 1.4 特 許 状 況 - 1 - - 2 - 1.5 起 原 又 は 発 見 の 経 緯 及 び 開 発 の 経 緯 FCT CT RH ... 1.5-1... 1.5-1... 1.5-1... 1.5-1... 1.5-2... 1.5-2... 1.5-2... 1.5-3...1.5-4... 1.5-5 i 10mg FCT 1 1 6 15 5mg CT 1 1 2 6 4mg CT
1.4 特 許 状 況 - 1 - - 2 - 1.5 起 原 又 は 発 見 の 経 緯 及 び 開 発 の 経 緯 FCT CT RH ... 1.5-1... 1.5-1... 1.5-1... 1.5-1... 1.5-2... 1.5-2... 1.5-2... 1.5-3...1.5-4... 1.5-5 i 10mg FCT 1 1 6 15 5mg CT 1 1 2 6 4mg CT
日本化学療法学会雑誌第54巻第S-1号
 β β Key words β Candida krusei Candida glabrata β I β Drugadministration Baselinecharacteristics Clinicalsymptom Mycologicaltests Plasmadrugconcn Adverseevents Laboratorytests ) -leadecg Clinicalphotograph
β β Key words β Candida krusei Candida glabrata β I β Drugadministration Baselinecharacteristics Clinicalsymptom Mycologicaltests Plasmadrugconcn Adverseevents Laboratorytests ) -leadecg Clinicalphotograph
50mg 75mg (1) H 3 C O OH HO H O O H OH SO H 3 Na H H N H 2 N NH OH H N O O H H O OH H 3 C H O O N H H H HO H NH CH 3 HN HO H OH O H OH H NH
 3240 14 8 8 50mg 75mg 13 6 25 1-(1) H 3 C O OH HO H O O H OH SO H 3 Na H H N H 2 N NH OH H N O O H H O OH H 3 C H O O N H H H HO H NH CH 3 HN HO H OH O H OH H NH O N O C 56 H 70 N 9 NaO 23 S 1292.26 5-[(1S,2S)-2-
3240 14 8 8 50mg 75mg 13 6 25 1-(1) H 3 C O OH HO H O O H OH SO H 3 Na H H N H 2 N NH OH H N O O H H O OH H 3 C H O O N H H H HO H NH CH 3 HN HO H OH O H OH H NH O N O C 56 H 70 N 9 NaO 23 S 1292.26 5-[(1S,2S)-2-
387 ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( )
 386 B B () () ( ) ( ) ( ) 387 ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) 388 ( ) ( ) ( ) 389 ( ) 2 ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) 390 ( ) ( ) ( ) () () ( ) Hot flush 391 ( ) ( ) ( ) 392 ( ( )
386 B B () () ( ) ( ) ( ) 387 ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) 388 ( ) ( ) ( ) 389 ( ) 2 ( ) ( ) ( ) ( ) ( ) ( ) ( ) ( ) 390 ( ) ( ) ( ) () () ( ) Hot flush 391 ( ) ( ) ( ) 392 ( ( )
37, 9-14, 2017 : cefcapene piperacillin 3 CT Clostridium difficile CD vancomycin CD 7 Clostridium difficile CD CD associate
 37, 9-14, 2017 : 36 2015 8 6 cefcapene piperacillin 3 CT Clostridium difficile CD vancomycin 20 1983 2016 3 CD 7 Clostridium difficile CD CD associated diarrhea : CDAD CD 1,2 PPI 3 1 36 33 2015 8 6 5 5
37, 9-14, 2017 : 36 2015 8 6 cefcapene piperacillin 3 CT Clostridium difficile CD vancomycin 20 1983 2016 3 CD 7 Clostridium difficile CD CD associated diarrhea : CDAD CD 1,2 PPI 3 1 36 33 2015 8 6 5 5
Rinku General Medical Center
 Rinku General Medical Center 4860 100 1620 Ann Intern Med. 1966; 64: 328 40 CHOP 63 1 83 J Clin Oncol. 1998; 16: 20065-69 Febrile Neutropenia IDSA(Infectious Diseases Society of America) 2002 Guidelines
Rinku General Medical Center 4860 100 1620 Ann Intern Med. 1966; 64: 328 40 CHOP 63 1 83 J Clin Oncol. 1998; 16: 20065-69 Febrile Neutropenia IDSA(Infectious Diseases Society of America) 2002 Guidelines
日本化学療法学会雑誌第54巻第S-1号
 β β β Key words β β I HP- -CD CHR RHC R R R R CHR R R R R RHC R R R CHR R R R RHC CHR R H CH CH Fig.. Structuralformulaofhydroxypropyl-β -cyclodextrin(hp-β -CD). CH n H β β β β Table-. Dosageandadministration(Singleadministrationstudy)
β β β Key words β β I HP- -CD CHR RHC R R R R CHR R R R R RHC R R R CHR R R R RHC CHR R H CH CH Fig.. Structuralformulaofhydroxypropyl-β -cyclodextrin(hp-β -CD). CH n H β β β β Table-. Dosageandadministration(Singleadministrationstudy)
日本医科大学医学会雑誌第8巻第1号
 Xenopus laevis in situ μ μ Xenopus laevis Xenopus laevis Xenopus laevis UGT1A1 UGT1A1 IL28B KRAS UGT1A1 UGT1A128 UGT UGT1A1 UGT1A128 UGT1A1 286 UGT1A1 UGT1A1 28 6 UGT UGT UGT1A128 UGT IL28B UGT1A1
Xenopus laevis in situ μ μ Xenopus laevis Xenopus laevis Xenopus laevis UGT1A1 UGT1A1 IL28B KRAS UGT1A1 UGT1A128 UGT UGT1A1 UGT1A128 UGT1A1 286 UGT1A1 UGT1A1 28 6 UGT UGT UGT1A128 UGT IL28B UGT1A1
O1-1 O1-2 O1-3 O1-4 O1-5 O1-6
 O1-1 O1-2 O1-3 O1-4 O1-5 O1-6 O1-7 O1-8 O1-9 O1-10 O1-11 O1-12 O1-13 O1-14 O1-15 O1-16 O1-17 O1-18 O1-19 O1-20 O1-21 O1-22 O1-23 O1-24 O1-25 O1-26 O1-27 O1-28 O1-29 O1-30 O1-31 O1-32 O1-33 O1-34 O1-35
O1-1 O1-2 O1-3 O1-4 O1-5 O1-6 O1-7 O1-8 O1-9 O1-10 O1-11 O1-12 O1-13 O1-14 O1-15 O1-16 O1-17 O1-18 O1-19 O1-20 O1-21 O1-22 O1-23 O1-24 O1-25 O1-26 O1-27 O1-28 O1-29 O1-30 O1-31 O1-32 O1-33 O1-34 O1-35
2 129-B*-006P 129-B*-007P C*-004P 129-D*-001P ADH B*-006P B*-007P C*-004P D*-001P Na
 2.7.4 2.7.4.1 2.7.4.1.1 11 ADH 1 ADH 1 4 7 129-A*-003 129-B*-001 129-E*-001P 129-G*-001 129-H*-002 129-H*-004 129-H*-005 132 242 4 129-B*-004 129-C*-005P 129-H*-003 129-H*-006 44 ADH ADH 129-C*-001P 16
2.7.4 2.7.4.1 2.7.4.1.1 11 ADH 1 ADH 1 4 7 129-A*-003 129-B*-001 129-E*-001P 129-G*-001 129-H*-002 129-H*-004 129-H*-005 132 242 4 129-B*-004 129-C*-005P 129-H*-003 129-H*-006 44 ADH ADH 129-C*-001P 16
 1 911 9001030 9:00 A B C D E F G H I J K L M 1A0900 1B0900 1C0900 1D0900 1E0900 1F0900 1G0900 1H0900 1I0900 1J0900 1K0900 1L0900 1M0900 9:15 1A0915 1B0915 1C0915 1D0915 1E0915 1F0915 1G0915 1H0915 1I0915
1 911 9001030 9:00 A B C D E F G H I J K L M 1A0900 1B0900 1C0900 1D0900 1E0900 1F0900 1G0900 1H0900 1I0900 1J0900 1K0900 1L0900 1M0900 9:15 1A0915 1B0915 1C0915 1D0915 1E0915 1F0915 1G0915 1H0915 1I0915
(1) (2) (1) (2) (1) Seg (2) Seg (3) Seg (4) Seg (1) (2) (3) ( ) (1) (PCA)...12
 (1)...109 (2)...110 (1) 28...111 (2) 28...113 (1) Seg...115 (2) Seg...117 (3) Seg...119 (4) Seg...120 (1)...122 (2)...122 (3) ( )...126 (1) (PCA)...127 (2) Draize...128 (1) Ames...129 2 in vitro...132
(1)...109 (2)...110 (1) 28...111 (2) 28...113 (1) Seg...115 (2) Seg...117 (3) Seg...119 (4) Seg...120 (1)...122 (2)...122 (3) ( )...126 (1) (PCA)...127 (2) Draize...128 (1) Ames...129 2 in vitro...132
日本化学療法学会雑誌第51巻第2号
 piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
untitled
 PGF 17 6 1 11 1 12 1 2 21 2 22 2 23 3 1 3 1 3 2 3 3 3 4 3 5 4 6 4 2 4 1 4 2 4 3 4 4 4 5 5 3 5 1 5 2 5 5 5 5 4 5 1 5 2 5 3 6 5 6 1 6 2 6 6 6 24 7 1 7 1 7 2 7 3 7 4 8 2 8 1 8 2 8 3 9 4 9 5 9 6 9 3 9 1 9
PGF 17 6 1 11 1 12 1 2 21 2 22 2 23 3 1 3 1 3 2 3 3 3 4 3 5 4 6 4 2 4 1 4 2 4 3 4 4 4 5 5 3 5 1 5 2 5 5 5 5 4 5 1 5 2 5 3 6 5 6 1 6 2 6 6 6 24 7 1 7 1 7 2 7 3 7 4 8 2 8 1 8 2 8 3 9 4 9 5 9 6 9 3 9 1 9
2009年133巻3号3月号.indb
 Folia Pharmacol. Jpn.133 1 2 Chiroscience UCB S RR-..% AA C IC C/A A/ A R- II/ III II/III Chiroscience UCB S 1 - E-mail: koji_taya@maruishi-pharm.co.jp Author: Koji Taya, Satoshi Shimizu Title: Levobupivacaine
Folia Pharmacol. Jpn.133 1 2 Chiroscience UCB S RR-..% AA C IC C/A A/ A R- II/ III II/III Chiroscience UCB S 1 - E-mail: koji_taya@maruishi-pharm.co.jp Author: Koji Taya, Satoshi Shimizu Title: Levobupivacaine
/ / A/ B 16/17 COPD 18mcg COPD COPD COPD 1
 11 1 -------------------- 19 205.117/205.128 14/15 205.126A/205.126B 16/17 COPD 18mcg COPD 11.1 11.1.1 COPD 100 19 205.227 COPD 1 FEV1.0 %FEV1.0 70% 1 FEV1.0 / FVC 70% 2 1 200 3 40 COPD 1 10mg ACE 1 1
11 1 -------------------- 19 205.117/205.128 14/15 205.126A/205.126B 16/17 COPD 18mcg COPD 11.1 11.1.1 COPD 100 19 205.227 COPD 1 FEV1.0 %FEV1.0 70% 1 FEV1.0 / FVC 70% 2 1 200 3 40 COPD 1 10mg ACE 1 1
E3200 BV FOLFOX4 FOLFOX4 PFS % 0.42, 0.65 p< log-rank % FOLFOX4 8.6% FOLFOX % FOLFOX4 259/ % FOL
 E3200 BV FOLFOX4 FOLFOX4 PFS 4.5 7.5 0.518 95% 0.42, 0.65 p
E3200 BV FOLFOX4 FOLFOX4 PFS 4.5 7.5 0.518 95% 0.42, 0.65 p
 5.3mg () 1 2SD 2 1.5SD 17 15 2 4 4 3 ( ) 瘙 3 嘔 気 嘔 吐 腹 痛 消 化 不 良 便 秘 4 24 p * p ** IGF-I (ng/ml) 77.12±60.19 238.97±143.60 161.85±113.30 [124.08, 199.63] (p
5.3mg () 1 2SD 2 1.5SD 17 15 2 4 4 3 ( ) 瘙 3 嘔 気 嘔 吐 腹 痛 消 化 不 良 便 秘 4 24 p * p ** IGF-I (ng/ml) 77.12±60.19 238.97±143.60 161.85±113.30 [124.08, 199.63] (p
nsg02-13/ky045059301600033210
 φ φ φ φ κ κ α α μ μ α α μ χ et al Neurosci. Res. Trpv J Physiol μ μ α α α β in vivo β β β β β β β β in vitro β γ μ δ μδ δ δ α θ α θ α In Biomechanics at Micro- and Nanoscale Levels, Volume I W W v W
φ φ φ φ κ κ α α μ μ α α μ χ et al Neurosci. Res. Trpv J Physiol μ μ α α α β in vivo β β β β β β β β in vitro β γ μ δ μδ δ δ α θ α θ α In Biomechanics at Micro- and Nanoscale Levels, Volume I W W v W
薬局におけるインシデント事例の集計・分析結果
 13 11 14 31 13 11 14 31 13 11 14 31 10 13 13 e-mail 18 4,000 13 14 H13.4.1 11.17 11.18 H14.3.31 13 14 31-1- 600 400 (18.4 17.7 17.0 16.0 15.2 8.2 1.0 2 11 10 12 15 3 1015203040 69.5 7.8 22.7 4 (42.5) 34.3
13 11 14 31 13 11 14 31 13 11 14 31 10 13 13 e-mail 18 4,000 13 14 H13.4.1 11.17 11.18 H14.3.31 13 14 31-1- 600 400 (18.4 17.7 17.0 16.0 15.2 8.2 1.0 2 11 10 12 15 3 1015203040 69.5 7.8 22.7 4 (42.5) 34.3
IF 1 MR 63 2 IF 10 3 IF 2IF IF IF 3IF A4 9 IF IF IF 11 1 IF IF 4IF IF MR IF IF MR Drug Safety Update IF
 2008 2 3 87269 IF19989 100g 700g 30g SucrosePovidoneIodine 1999 3 15 1999 7 9 2000 5 IF 2008 2 IF 1 MR 63 2 IF 10 3 IF 2IF IF IF 3IF A4 9 IF IF IF 11 1 IF IF 4IF IF MR IF IF MR Drug Safety Update IF 1981
2008 2 3 87269 IF19989 100g 700g 30g SucrosePovidoneIodine 1999 3 15 1999 7 9 2000 5 IF 2008 2 IF 1 MR 63 2 IF 10 3 IF 2IF IF IF 3IF A4 9 IF IF IF 11 1 IF IF 4IF IF MR IF IF MR Drug Safety Update IF 1981
 200 30 984 1 1854 1713 1000 1008 ED ADL/QOL The European Working Group on Sarcopenia in Older People EWGSOP Asian Working Group for Sarcopenia AWGS D C Zhang, 2006 C Imamura, 2008 ATP Imamura, 2009 Imamura,
200 30 984 1 1854 1713 1000 1008 ED ADL/QOL The European Working Group on Sarcopenia in Older People EWGSOP Asian Working Group for Sarcopenia AWGS D C Zhang, 2006 C Imamura, 2008 ATP Imamura, 2009 Imamura,
untitled
 2303 16 2 9 1 14 5 31 (423) 1 (4), (6) C55H84ClN17O21S3 HCl (BLM-A2) 1487.47 11 2 1 4 104 2 14 5 31 [ ] ( ) ( ) 1. 15mg 30mg( ) 5 20mL 1 5mg( ) 2. 3 15mg 30mg( ) 5mL 1mg( ) 1mL 3. 5mg 15mg( ) 4. 1 2 1
2303 16 2 9 1 14 5 31 (423) 1 (4), (6) C55H84ClN17O21S3 HCl (BLM-A2) 1487.47 11 2 1 4 104 2 14 5 31 [ ] ( ) ( ) 1. 15mg 30mg( ) 5 20mL 1 5mg( ) 2. 3 15mg 30mg( ) 5mL 1mg( ) 1mL 3. 5mg 15mg( ) 4. 1 2 1
) Meropenem Chemotherapy (Tokyo) ) panipenem/betamipron Chemotherapy (Tokyo) ) Meropenem Chemotherapy (Toky
 2.7 2.7.5 1) 199846410-437 2) UTI ( )UTI ( 3 )Chemotherapy (Tokyo) 1986 34408-441 3) () 199745762-778 4) 1992 5) 199543 6) Chemotherapy (Tokyo) 199139687-689 7) Imipenem/Cilastatin sodium (MK-0787/MK-0791)
2.7 2.7.5 1) 199846410-437 2) UTI ( )UTI ( 3 )Chemotherapy (Tokyo) 1986 34408-441 3) () 199745762-778 4) 1992 5) 199543 6) Chemotherapy (Tokyo) 199139687-689 7) Imipenem/Cilastatin sodium (MK-0787/MK-0791)
 IMUSERA 3 3 4 4 5 5 5 5 6 1 1 12 18 25 25 31 31 31 31 32 32 37 43 43 45 48 48 48 48 49 5 1 2 3 4 3 4 1.56mg.5mg D- 3 15.9mm 5.8mm.96g FTY.5mg FTY.5mg OH NH2 OH H3C HCI 5 6 7 BCG Torsades de pointes a 2
IMUSERA 3 3 4 4 5 5 5 5 6 1 1 12 18 25 25 31 31 31 31 32 32 37 43 43 45 48 48 48 48 49 5 1 2 3 4 3 4 1.56mg.5mg D- 3 15.9mm 5.8mm.96g FTY.5mg FTY.5mg OH NH2 OH H3C HCI 5 6 7 BCG Torsades de pointes a 2
スライド タイトルなし
 2395 20 3 2008 5 16 SHIN NIPPON BIOMEDICAL LABORATORIES,LTD. 1 2 CONTENTS. 20 3 CFO. 2008 CEO... Translational Translational 3 . 20 3 CFO 4 20 3 P/L 19 3 20 3 17,289 19,500 19,647 +2,358 +147 1,125 1,453
2395 20 3 2008 5 16 SHIN NIPPON BIOMEDICAL LABORATORIES,LTD. 1 2 CONTENTS. 20 3 CFO. 2008 CEO... Translational Translational 3 . 20 3 CFO 4 20 3 P/L 19 3 20 3 17,289 19,500 19,647 +2,358 +147 1,125 1,453
untitled
 1% 1 mg 1 mg 2 CTD 6 Section 2.6.1 Introduction 2.6.1 H 1 descarboethoxyloratadinesch 34117 7.9 SCH 34117 2.6.1-1 O OC 2 H 5 Cl N N JAN Loratadine (JANINN) ethyl 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]
1% 1 mg 1 mg 2 CTD 6 Section 2.6.1 Introduction 2.6.1 H 1 descarboethoxyloratadinesch 34117 7.9 SCH 34117 2.6.1-1 O OC 2 H 5 Cl N N JAN Loratadine (JANINN) ethyl 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]
 1) Steinbrocker, O.: The shoulder-hand syndrome in reflex dystrophy of the upper ex- tremity, Ann. Intern. Med., 29: 22, 1948. 5) Greenfield, A. D. M., Whitney, R. J. and Mowbray, J. F.: Methods for the
1) Steinbrocker, O.: The shoulder-hand syndrome in reflex dystrophy of the upper ex- tremity, Ann. Intern. Med., 29: 22, 1948. 5) Greenfield, A. D. M., Whitney, R. J. and Mowbray, J. F.: Methods for the
日本化学療法学会雑誌第58巻第2号
 Key words β Candida Table1. MCFGCPAstudygroup Investigator (representative) YoshitsuguMiyazaki NaoyukiMiyashita RyoichiAmitani KenjiOgawa AtsuyukiKurashima ToshiroKiguchi MichiakiMishima YuichiInoue HiroshiSaito
Key words β Candida Table1. MCFGCPAstudygroup Investigator (representative) YoshitsuguMiyazaki NaoyukiMiyashita RyoichiAmitani KenjiOgawa AtsuyukiKurashima ToshiroKiguchi MichiakiMishima YuichiInoue HiroshiSaito
1 12 *1 *2 (1991) (1992) (2002) (1991) (1992) (2002) 13 (1991) (1992) (2002) *1 (2003) *2 (1997) 1
 2005 1 1991 1996 5 i 1 12 *1 *2 (1991) (1992) (2002) (1991) (1992) (2002) 13 (1991) (1992) (2002) *1 (2003) *2 (1997) 1 2 13 *3 *4 200 1 14 2 250m :64.3km 457mm :76.4km 200 1 548mm 16 9 12 589 13 8 50m
2005 1 1991 1996 5 i 1 12 *1 *2 (1991) (1992) (2002) (1991) (1992) (2002) 13 (1991) (1992) (2002) *1 (2003) *2 (1997) 1 2 13 *3 *4 200 1 14 2 250m :64.3km 457mm :76.4km 200 1 548mm 16 9 12 589 13 8 50m
ŁRŁ¸•ñŁŁŁ‘
 16 11 5 25mg 14 11 18 1 25mg 1 150,000 934 1 2 : 1-235-tumor necrosis factor receptor (human) fusion protein with 236-467-immunoglobulin G1 (human γ1-chain Fc fragment), dimmer : : 1 235 236 467 G 1 γ1
16 11 5 25mg 14 11 18 1 25mg 1 150,000 934 1 2 : 1-235-tumor necrosis factor receptor (human) fusion protein with 236-467-immunoglobulin G1 (human γ1-chain Fc fragment), dimmer : : 1 235 236 467 G 1 γ1
SBP hospitalist network.key
 1 Treatment and Prophylaxis of Spontaneous Bacterial Peritonitis (1); (2), (3). (1) (2) (3) :, :, 2 Clinical Question 1 72...,.,,.,... 3. CT..,. 4? SBP? SBP. 5 Clinical Question SBP? SBP,? 6 SBP (Spontaneous
1 Treatment and Prophylaxis of Spontaneous Bacterial Peritonitis (1); (2), (3). (1) (2) (3) :, :, 2 Clinical Question 1 72...,.,,.,... 3. CT..,. 4? SBP? SBP. 5 Clinical Question SBP? SBP,? 6 SBP (Spontaneous
Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution of female patients with blad
 Key words: Female with bladder irritability, Subjective symptoms, Pyuria, Bacteriuria Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution
Key words: Female with bladder irritability, Subjective symptoms, Pyuria, Bacteriuria Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution
( ) FAS87 FAS FAS87 v = 1 i 1 + i
 ( ) ( 7 6 ) ( ) 1 6 1 18 FAS87 FAS87 7 1 FAS87 v = 1 i 1 + i 10 14 6 6-1 - 7 73 2 N (m) N L m a N (m) L m a N m a (m) N 73 9 99 18 4-2 - 4 143 2 145 3 37 4 37 4 40 6 40 6 41 10 41 10 13 10 14 4 24 3 145
( ) ( 7 6 ) ( ) 1 6 1 18 FAS87 FAS87 7 1 FAS87 v = 1 i 1 + i 10 14 6 6-1 - 7 73 2 N (m) N L m a N (m) L m a N m a (m) N 73 9 99 18 4-2 - 4 143 2 145 3 37 4 37 4 40 6 40 6 41 10 41 10 13 10 14 4 24 3 145
400 46 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 Dec. 2012 LVFX 100 mg 3 / 7 150 mg 2 / 7 2 2006 2008 9 LVFX PK PD 2009 7 100 mg 1 3 500 mg 1 1 AUC/MIC
 Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
‰Łflç‘ÇŁ\”ƒ(’FŁª›ð)-A4
 1 14163 1611 (2002) 32007 3 3 196 2 1416 ( ) Medsger modified Rodnan total skin thickness score EBM placebo control ACEEBM EBM 3 1611 4 5 (Systemic Sclerosis : SSc) 1980 (1) 1) 200310 (2) 2003 Scl-70 1
1 14163 1611 (2002) 32007 3 3 196 2 1416 ( ) Medsger modified Rodnan total skin thickness score EBM placebo control ACEEBM EBM 3 1611 4 5 (Systemic Sclerosis : SSc) 1980 (1) 1) 200310 (2) 2003 Scl-70 1
1) a) b) CRP c) d) e) a,b b,c c,d d,e a,e b,d 2) CKD a) ACE ARB b) 2mg/dL ACE ARB c) 2mg/dL d) e) ACE ARB a,b b,c c,d d,e a,e b,d 3) a) 130/85mmHg b)
 2010.1.13 1) 4) 2 18 1) 1 2 3 4 2) 1 2 85 3 3) 1 metabolizer 2 metabolizer 3 metabolizer 4 metabolizer 4) CYP 1 2 metabolizer 3 metabolizer 4 5 6 4 7 2 5 (PPI) 1 2 PPI 2 3 2 3 3 3 3 PPI 3 1 ph 4 5 1) 5)
2010.1.13 1) 4) 2 18 1) 1 2 3 4 2) 1 2 85 3 3) 1 metabolizer 2 metabolizer 3 metabolizer 4 metabolizer 4) CYP 1 2 metabolizer 3 metabolizer 4 5 6 4 7 2 5 (PPI) 1 2 PPI 2 3 2 3 3 3 3 PPI 3 1 ph 4 5 1) 5)
Endocrinological and statistical analysis of ovarian function in hysterectomized patients Masaya HAYAFUJI, Kazuaki KATAYAMA and Matsuto MOCHIZUKI Depa
 Endocrinological and statistical analysis of ovarian function in hysterectomized patients Masaya HAYAFUJI, Kazuaki KATAYAMA and Matsuto MOCHIZUKI Department of Obstetrics and Gynecology, Kobe University
Endocrinological and statistical analysis of ovarian function in hysterectomized patients Masaya HAYAFUJI, Kazuaki KATAYAMA and Matsuto MOCHIZUKI Department of Obstetrics and Gynecology, Kobe University
 2 1 7 - TALK ABOUT 21 μ TALK ABOUT 21 Ag As Se 2. 2. 2. Ag As Se 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 Sb Ga Te 2. Sb 2. Ga 2. Te 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 1 2 3 4
2 1 7 - TALK ABOUT 21 μ TALK ABOUT 21 Ag As Se 2. 2. 2. Ag As Se 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 Sb Ga Te 2. Sb 2. Ga 2. Te 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 1 2 3 4
アクテムラインタビューフォーム
 2009 2 6 876399 IF 1998 9 FAX 80mg 1 4mL 80mg 200mg 1 10mL200mg 400mg 1 20mL400mg JAN TocilizumabGenetical RecombinationJAN 200mg 80400mg 2007 9 10 2008 4 16 2007 12 21 2008 6 13 2005 6 13 2008 6 13 2009
2009 2 6 876399 IF 1998 9 FAX 80mg 1 4mL 80mg 200mg 1 10mL200mg 400mg 1 20mL400mg JAN TocilizumabGenetical RecombinationJAN 200mg 80400mg 2007 9 10 2008 4 16 2007 12 21 2008 6 13 2005 6 13 2008 6 13 2009
Vol. 36, Special Issue, S 3 S 18 (2015) PK Phase I Introduction to Pharmacokinetic Analysis Focus on Phase I Study 1 2 Kazuro Ikawa 1 and Jun Tanaka 2
 Vol. 36, Special Issue, S 3 S 18 (2015) PK Phase I Introduction to Pharmacokinetic Analysis Focus on Phase I Study 1 2 Kazuro Ikawa 1 and Jun Tanaka 2 1 2 1 Department of Clinical Pharmacotherapy, Hiroshima
Vol. 36, Special Issue, S 3 S 18 (2015) PK Phase I Introduction to Pharmacokinetic Analysis Focus on Phase I Study 1 2 Kazuro Ikawa 1 and Jun Tanaka 2 1 2 1 Department of Clinical Pharmacotherapy, Hiroshima
6„”“ƒ„û−G33
 C O N T E N T S 2,706 3,183 3,957 0100101 22 10 21 1,414 1,663 2,250 0601603102 2000
C O N T E N T S 2,706 3,183 3,957 0100101 22 10 21 1,414 1,663 2,250 0601603102 2000
グリセオール注インタビューフォーム
 2006 8 3 873999 871319 IF 1998 9 FAX 1 200mL 300mL 500mL 20g 30g 50g 10g 15g 25g 1.8g 2.7g 4.5g 0.9W/V 200mL500mL 1979 3 13 2004 7 9 1979 5 15 2006 8 300mL 1979 3 13 2004 7 9 1982 11 1 IF 1 MR 63 IF 10
2006 8 3 873999 871319 IF 1998 9 FAX 1 200mL 300mL 500mL 20g 30g 50g 10g 15g 25g 1.8g 2.7g 4.5g 0.9W/V 200mL500mL 1979 3 13 2004 7 9 1979 5 15 2006 8 300mL 1979 3 13 2004 7 9 1982 11 1 IF 1 MR 63 IF 10
(1.2) T D = 0 T = D = 30 kn 1.2 (1.4) 2F W = 0 F = W/2 = 300 kn/2 = 150 kn 1.3 (1.9) R = W 1 + W 2 = = 1100 N. (1.9) W 2 b W 1 a = 0
 1 1 1.1 1.) T D = T = D = kn 1. 1.4) F W = F = W/ = kn/ = 15 kn 1. 1.9) R = W 1 + W = 6 + 5 = 11 N. 1.9) W b W 1 a = a = W /W 1 )b = 5/6) = 5 cm 1.4 AB AC P 1, P x, y x, y y x 1.4.) P sin 6 + P 1 sin 45
1 1 1.1 1.) T D = T = D = kn 1. 1.4) F W = F = W/ = kn/ = 15 kn 1. 1.9) R = W 1 + W = 6 + 5 = 11 N. 1.9) W b W 1 a = a = W /W 1 )b = 5/6) = 5 cm 1.4 AB AC P 1, P x, y x, y y x 1.4.) P sin 6 + P 1 sin 45
本文/開催および演題募集のお知らせ
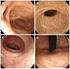
 86 QOL S Masson Irritable Bowel Syndrome IBS Visual Analog Scale VAS IBS MRI S pelvic side wall W pelvic side wall PDS figure 過敏性腸炎様の症状を呈した直腸子宮内膜症の症例 87 図1 術前 MRI ゼリー法の結果 1 症例1の術前所見 症例の術前所見では に直腸子宮内膜症を疑う
86 QOL S Masson Irritable Bowel Syndrome IBS Visual Analog Scale VAS IBS MRI S pelvic side wall W pelvic side wall PDS figure 過敏性腸炎様の症状を呈した直腸子宮内膜症の症例 87 図1 術前 MRI ゼリー法の結果 1 症例1の術前所見 症例の術前所見では に直腸子宮内膜症を疑う
 gtf Wnt5a-Ror2 IL-8 in vitro Ozony Tron P. gingivalis SOD ELISA Location of main occluding areas and masticatory ability in patients with reduced occlusal support 12-Methacryloyloxydodecylpyridinium bromide
gtf Wnt5a-Ror2 IL-8 in vitro Ozony Tron P. gingivalis SOD ELISA Location of main occluding areas and masticatory ability in patients with reduced occlusal support 12-Methacryloyloxydodecylpyridinium bromide
1 2 1.2 1.2.1 =PAS.. =.. =.... 1% 01% ] 1.2.2 = = = = 4 (A/A 2 / /B ) 1 8 2n / 16 32 2n n 64 n 64 n ml /ml AI ml 50 5 10 0.5 0.25-0.5 30 1 30 1 0.2-0.
![1 2 1.2 1.2.1 =PAS.. =.. =.... 1% 01% ] 1.2.2 = = = = 4 (A/A 2 / /B ) 1 8 2n / 16 32 2n n 64 n 64 n ml /ml AI ml 50 5 10 0.5 0.25-0.5 30 1 30 1 0.2-0. 1 2 1.2 1.2.1 =PAS.. =.. =.... 1% 01% ] 1.2.2 = = = = 4 (A/A 2 / /B ) 1 8 2n / 16 32 2n n 64 n 64 n ml /ml AI ml 50 5 10 0.5 0.25-0.5 30 1 30 1 0.2-0.](/thumbs/40/22237015.jpg) 1 1 1 1.1 1.1.1 3 : 0: + : +0 : : } 40 40 40 = 3 35 35 (38 )? +0+7 30 35-0+0 38 38 +30 5 30 30 +20 16 16 1. [ ] 2. 3. 4. + = etc 1.1.2 Ru 1 2 1.2 1.2.1 =PAS.. =.. =.... 1% 01% ] 1.2.2 = = = = 4 (A/A 2
1 1 1 1.1 1.1.1 3 : 0: + : +0 : : } 40 40 40 = 3 35 35 (38 )? +0+7 30 35-0+0 38 38 +30 5 30 30 +20 16 16 1. [ ] 2. 3. 4. + = etc 1.1.2 Ru 1 2 1.2 1.2.1 =PAS.. =.. =.... 1% 01% ] 1.2.2 = = = = 4 (A/A 2
Contents 4 06 IP2 41 Thermo Scientific Nalgene Nalgene
 Nalgene & Packaging plastic Bottles & Vials Contents 4 06 IP2 41 Thermo Scientific Nalgene 5 07 42 8 08 43 9 09 45 11 10 46 Nalgene 12 11 50 13 12 53 01 20 13 61 02 28 14 64 03 33 15 66 04 37 16 / 67 05
Nalgene & Packaging plastic Bottles & Vials Contents 4 06 IP2 41 Thermo Scientific Nalgene 5 07 42 8 08 43 9 09 45 11 10 46 Nalgene 12 11 50 13 12 53 01 20 13 61 02 28 14 64 03 33 15 66 04 37 16 / 67 05
研修コーナー
 l l l l l l l l l l l α α β l µ l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l
l l l l l l l l l l l α α β l µ l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l l
 Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
rule2018.dvi
 30 2018 30 4 21 ( RC ) 1. 2. 2018 6 16 ( ) 2018 6 17 ( ) : (URL: http://www.kagakukan.sendai-c.ed.jp) 981 0903 4 1 Tel: 022-276-2201, Fax: 022-276-2204 3. : : : 2018 5 11 ( ) (ID ) 2018 5 14 ( ) () 2018
30 2018 30 4 21 ( RC ) 1. 2. 2018 6 16 ( ) 2018 6 17 ( ) : (URL: http://www.kagakukan.sendai-c.ed.jp) 981 0903 4 1 Tel: 022-276-2201, Fax: 022-276-2204 3. : : : 2018 5 11 ( ) (ID ) 2018 5 14 ( ) () 2018
 1 ) Birnberg et al; Am. J. Obst. Gyn., 54 :88E 1947. 2) Bromberg, Y.M. & Bercovici, B. : Acta Endocrin., 7; 71, 1956. 23 : 33, 1956; Fertil. and Steril. 3) Fluhmann, C. F. : The management of menstrual
1 ) Birnberg et al; Am. J. Obst. Gyn., 54 :88E 1947. 2) Bromberg, Y.M. & Bercovici, B. : Acta Endocrin., 7; 71, 1956. 23 : 33, 1956; Fertil. and Steril. 3) Fluhmann, C. F. : The management of menstrual
A B 1.A B A B 2.A B A B 3.A
 1 1. 2.WHO 3. 4. 5. 2 2.0mg/kg / 1. 2. 3. 4. 5. 3 1. 90 2. 3. 4. 5. 4 1. 2. 3. 4. 5. 1 21 5 1. 2. 2050 61 3. 1 4. 5. 6 1. ----------- 2. ---- 3. ------ 4. -------- 5. --------- 7 A B 1.A B------------------A
1 1. 2.WHO 3. 4. 5. 2 2.0mg/kg / 1. 2. 3. 4. 5. 3 1. 90 2. 3. 4. 5. 4 1. 2. 3. 4. 5. 1 21 5 1. 2. 2050 61 3. 1 4. 5. 6 1. ----------- 2. ---- 3. ------ 4. -------- 5. --------- 7 A B 1.A B------------------A
審査報告書(案)
 19 83 7 18 628 1 19 8 3 7 18 6 28 30 MI 31 360 21700BZY00247000 5mm CEA 59 mm 2 50% 80 1. 2. 3 19 8 3 [ ] 7 [ ] [ ] [ ] [ ] 18 6 28 [ ] CEA 49mm 50 80 2 4 carotid endarterectomy CEA 1,2 CEA ECST 3 NASCET
19 83 7 18 628 1 19 8 3 7 18 6 28 30 MI 31 360 21700BZY00247000 5mm CEA 59 mm 2 50% 80 1. 2. 3 19 8 3 [ ] 7 [ ] [ ] [ ] [ ] 18 6 28 [ ] CEA 49mm 50 80 2 4 carotid endarterectomy CEA 1,2 CEA ECST 3 NASCET
indd
 Molecular Targeted Therapy for Lung Cancer from a Pharmaceutical Point of Viewe Naho SASAKI epidermal growth factor receptor tyrosine kinase inhibitor :EGFR-TKI vascular endothelial growth factor:vegf
Molecular Targeted Therapy for Lung Cancer from a Pharmaceutical Point of Viewe Naho SASAKI epidermal growth factor receptor tyrosine kinase inhibitor :EGFR-TKI vascular endothelial growth factor:vegf
1999年3月作成(新様式第1版)
 200622 19989 871313 1mL4mg JAN oxybuprocaine hydrochloridejan 2002621 20037 4 19667 1 FAX 2005 10 29607 05 1 63 () 103 2 3 49 111 4 Drug Safety Update 1. -------------------------------------1 2. -----------------------1
200622 19989 871313 1mL4mg JAN oxybuprocaine hydrochloridejan 2002621 20037 4 19667 1 FAX 2005 10 29607 05 1 63 () 103 2 3 49 111 4 Drug Safety Update 1. -------------------------------------1 2. -----------------------1
日本化学療法学会雑誌第59巻第5号
 Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
BUN, CRP K mg/ cm, 49.6 kg, BMI /72 mmhg, 92/ Hb 6.7 g/dl PT-INR CT 1 MRI 2a, b T1 T2 T1 MRI
 20 2 2015 1 2 3 85 K CT Abstract A case of renal subcapsular hematoma resulting from trigger point injection under excessive effect of anticoagulant YAMANE Tateki, UMEDA Akira and SHIMAO Hitoshi An 85-year-old
20 2 2015 1 2 3 85 K CT Abstract A case of renal subcapsular hematoma resulting from trigger point injection under excessive effect of anticoagulant YAMANE Tateki, UMEDA Akira and SHIMAO Hitoshi An 85-year-old
日本呼吸器学会雑誌第44巻第1号
 β β l β β Table1 Laboratorydataonadmission Hematology WBC 9,910/μl neut 72.4% lym 20.2% eos 0.9% mon 6.1% baso 0.4% RBC 491 10 4 /μl Hb 16.8g/dl Ht 48.3% PLT 29.8 10 4 /μl ESR 3mm/hr Biochemistry TP Alb
β β l β β Table1 Laboratorydataonadmission Hematology WBC 9,910/μl neut 72.4% lym 20.2% eos 0.9% mon 6.1% baso 0.4% RBC 491 10 4 /μl Hb 16.8g/dl Ht 48.3% PLT 29.8 10 4 /μl ESR 3mm/hr Biochemistry TP Alb
本文/一般演題_吉田
 184 quality of lifeqol mmhg Hb gdl CA Uml Niveau Hb Hb gdl RCC CT MRI T 185 CT A B MRI A:T B:T Douglas red spot S ml RCC 186 A B mg CAUml S MRI A B 187 188 S mg Sampson JA. Intestinal adenomas of endometriosis
184 quality of lifeqol mmhg Hb gdl CA Uml Niveau Hb Hb gdl RCC CT MRI T 185 CT A B MRI A:T B:T Douglas red spot S ml RCC 186 A B mg CAUml S MRI A B 187 188 S mg Sampson JA. Intestinal adenomas of endometriosis
「 (会の名称) 」のご案内
 千葉県病院薬剤師会北部支部会員各位 16 年 2 月吉日 千葉県病院薬剤師会北部支部 学術講演会 北部支部長土谷隆紀謹啓時下ますますご清祥の段 お慶び申し上げます さてこの度 千葉県病院薬剤師会北部支部 学術講演会を下記の通り 開催させて頂く事となりました つきましてはご多忙中のこととは存じますが万障繰り合わせの上 ご出席賜りますようにお願い申し上げます 謹白記 日時 : 平成 28 年 4 月 15
千葉県病院薬剤師会北部支部会員各位 16 年 2 月吉日 千葉県病院薬剤師会北部支部 学術講演会 北部支部長土谷隆紀謹啓時下ますますご清祥の段 お慶び申し上げます さてこの度 千葉県病院薬剤師会北部支部 学術講演会を下記の通り 開催させて頂く事となりました つきましてはご多忙中のこととは存じますが万障繰り合わせの上 ご出席賜りますようにお願い申し上げます 謹白記 日時 : 平成 28 年 4 月 15
,328 C 6426 H 9900 N 1700 O 2008 S , ,
 2458 13 4 24 1 11 9 17 1,328 C 6426 H 9900 N 1700 O 2008 S 44 145,000 148,000 10 11 27 2 13 4 24 11 9 17 (1) CD20 B CD20 (2) infusion reaction infusion-associated symptom CD20 B 1 375mg/m 2 1 4 3 Heavy
2458 13 4 24 1 11 9 17 1,328 C 6426 H 9900 N 1700 O 2008 S 44 145,000 148,000 10 11 27 2 13 4 24 11 9 17 (1) CD20 B CD20 (2) infusion reaction infusion-associated symptom CD20 B 1 375mg/m 2 1 4 3 Heavy
 1)Malmivaara A, Hakkinen U, et al: The treatment of acute low back pain - bed rest, exercise, or ordinary activity? N Engl J Med 1995;332:251-5 2)Latash ML: Spectral analysis of the electromyogram (EMG)
1)Malmivaara A, Hakkinen U, et al: The treatment of acute low back pain - bed rest, exercise, or ordinary activity? N Engl J Med 1995;332:251-5 2)Latash ML: Spectral analysis of the electromyogram (EMG)
_0212_68<5A66><4EBA><79D1>_<6821><4E86><FF08><30C8><30F3><30DC><306A><3057><FF09>.pdf

ec6
 Yamagata Journal of Health Sciences, Vol. 8, 2005 Chikako SAITOU, Miharu NISHIWAKI This study has been undertaken to make clear the relationship of menstruation pattern to self-awareness, premenstrual
Yamagata Journal of Health Sciences, Vol. 8, 2005 Chikako SAITOU, Miharu NISHIWAKI This study has been undertaken to make clear the relationship of menstruation pattern to self-awareness, premenstrual



















