日本化学療法学会雑誌第60巻第4号
|
|
|
- さや あきます
- 4 years ago
- Views:
Transcription
1 Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Key words β Streptococcus pneumoniae Haemophilus influenzae
2 S. pneumoniae H. influenzae μ μ S. pneumoniae H. influenzae S. pneumoniae H. influenzae μ H. influenzae H. influenzae H. influenzae H. influenzae S. pneumoniae H. influenzae I μ
3 S. pneumoniae H. influenzae γ
4 Consent obtained: 116 Pneumonia Compliance analysis: 115 Pneumonia Total excluded: 1 Pneumonia Safety analysis: 115 Pneumonia Efficacy analysis: 106 Pneumonia Total excluded: 9 Pneumonia Fig. 1. Analysis population. S. pneumoniae H. influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis Streptococcus pyogenes S. pneumoniae H. influenzae S. pyogenes Staphylococcus aureus M. catarrhalis
5 Table 1. Patient profiles Item Gender Age (yr) Body weight (kg) Severity of infection Underlying disease and/or complication Showing no response to previous treatment Recurrent Pneumonia (n 14) (n 79) Patients (%) (n 13) Total (n 106) Male 6 (42.9) 51 (64.6) 5 (38.5) 62 (58.5) Female 8 (57.1) 28 (35.4) 8 (61.5) 44 (41.5) (0.0) 16 (20.3) 4 (30.8) 20 (18.9) 36 8 (57.1) 26 (32.9) 8 (61.5) 42 (39.6) (42.9) 37 (46.8) 1 (7.7) 44 (41.5) (14.3) 10 (12.7) 1 (7.7) 13 (12.3) (85.7) 58 (73.4) 9 (69.2) 79 (74.5) (0.0) 10 (12.7) 3 (23.1) 13 (12.3) 30 0 (0.0) 1 (1.3) 0 (0.0) 1 (0.9) Mild 6 (42.9) 0 (0.0) 0 (0.0) 6 (5.7) Moderate 8 (57.1) 24 (30.4) 6 (46.2) 38 (35.8) Severe 0 (0.0) 55 (69.6) 7 (53.8) 62 (58.5) No 7 (50.0) 7 (8.9) 8 (61.5) 22 (20.8) Yes 7 (50.0) 72 (91.1) 5 (38.5) 84 (79.2) No 14 (100.0) 74 (93.7) 11 (84.6) 99 (93.4) Yes 0 (0.0) 5 (6.3) 2 (15.4) 7 (6.6) No 71 (89.9) 6 (46.2) Yes 8 (10.1) 7 (53.8) Item Causative organism (n 3) Pneumonia Table 2. Causative organisms before administration presumed causative organism (n 14) (n 39) Strains (%) (n 12) Causative organism (n 54) Total presumed causative organism (n 65) S. pneumoniae 0 (0/3) 2 (14.3) 11 (28.2) 2 (16.7) 13 (24.1) 15 (23.1) H. influenzae 3 (3/3) 12 (85.7) 21 (53.8) 9 (75.0) 33 (61.1) 42 (64.6) M. catarrhalis 0 (0/3) 0 (0.0) 4 (10.3) 0 (0.0) 4 (7.4) 4 (6.2) S. pyogenes 0 (0/3) 0 (0.0) 3 (7.7) 1 (8.3) 4 (7.4) 4 (6.2) II
6 CLSI (PCG) Resistance Genotype of penicillin-binding proteins CLSI (CAM) Table 3. S. pneumoniae resistance distribution Pneumonia (n 2) (n 11) Strains (%) (n 2) Total (n 15) S 0 (0/2) 5 (45.5) 1 (1/2) 6 (40.0) I 2 (2/2) 4 (36.4) 1 (1/2) 7 (46.7) R 0 (0/2) 2 (18.2) 0 (0/2) 2 (13.3) gpisp (2x) 0 (0/2) 5 (45.5) 1 (1/2) 6 (40.0) gpisp (1a 2x) 0 (0/2) 1 (9.1) 0 (0/2) 1 (6.7) gpisp (2x 2b) 0 (0/2) 1 (9.1) 1 (1/2) 2 (13.3) gpisp (2b) 0 (0/2) 0 (0.0) 0 (0/2) 0 (0.0) gprsp (1a 2x 2b) 2 (2/2) 4 (36.4) 0 (0/2) 6 (40.0) S 0 (0/2) 0 (0.0) 0 (0/2) 0 (0.0) I 0 (0/2) 0 (0.0) 0 (0/2) 0 (0.0) R 2 (2/2) 11 (100.0) 2 (2/2) 15 (100.0) mefa 0 (0/2) 1 (9.1) 0 (0/2) 1 (6.7) Macrolide-resistance genotype ermb 0 (0/2) 9 (81.8) 2 (2/2) 11 (73.3) mefa ermb 2 (2/2) 1 (9.1) 0 (0/2) 3 (20.0) S: susceptible PCG MIC: 0.06 g/ml, I: intermediate PCG MIC: g/ml, R: resistant PCG MIC: 2 g/ml CLSI (ABPC) Resistance Table 4. H. influenzae resistance distribution Pneumonia (n 12) (n 21) Strains (%) (n 9) Total (n 42) S 6 (50.0) 7 (33.3) 3 (33.3) 16 (38.1) I 1 (8.3) 1 (4.8) 2 (22.2) 4 (9.5) R 5 (41.7) 13 (61.9) 4 (44.4) 22 (52.4) gblnas 3 (25.0) 4 (19.0) 2 (22.2) 9 (21.4) glow-blnar 2 (16.7) 3 (14.3) 1 (11.1) 6 (14.3) Genotype of penicillin-binding proteins gblnar 7 (58.3) 12 (57.1) 6 (66.7) 25 (59.5) gblpacr-ii 0 (0.0) 2 (9.5) 0 (0.0) 2 (4.8) S: susceptible CAM MIC: 0.25 g/ml, I: intermediate CAM MIC: 0.5 g/ml, R: resistant CAM MIC: 1 g/ml S. pneumoniae H. influenzae M. catarrhalis S. pyogenes
7 Tested drug No. of strains Range ( g/ml) Table 5. S. pneumoniae and H. influenzae drug susceptibility S. pneumoniae H. influenzae MIC50 ( g/ml) MIC90 ( g/ml) No. of strains Range ( g/ml) MIC50 ( g/ml) MIC90 ( g/ml) CDTR TBPM PCG AMPC ABPC CFDN CFPN CAM CDTR: cefditoren, TBPM: tebipenem, PCG: penicillin, AMPC: amoxicillin, ABPC: ampicillin, CFDN: cefdinir, CFPN: cefcapene, CAM: clarithromycin Item Pneumonia (n 14) Table 6. Clinical efficacy and cure rate Patients (%) (n 79) (n 13) Total (n 106) Efficacy (%) (97/106) Excellent 6 (42.9) 36 (45.6) 1 (7.7) Good 8 (57.1) 35 (44.3) 11 (84.6) Fair 0 (0.0) 6 (7.6) 1 (7.7) Poor 0 (0.0) 2 (2.5) 0 (0.0) Cure rate (%) (86/100) Cure Yes 12 (85.7) 65 (82.3) 9 (69.2) No 0 (0.0) 11 (13.9) 3 (23.1) S. pneumoniae H. influenzae S. pneumoniae H. influenzae S. pneumoniae S. pneumoniae pbp2x g g g pbp2x mefa ermb H. influenzae H. influenzae g g g g S. pneumoniae H. influenzae S. pneumoniae μ μ μ μ μ μ μ S. pneu-
8 Table 7. Clinical efficacy in recurrent or no response to previous treatment (n 79) Severity of infection Item Showing no response to previous treatment Recurrent Recurrent or Showing no response to previous treatment (n 13) Severity of infection Showing no response to previous treatment Recurrent Recurrent or Showing no response to previous treatment No. Patients (%) Excellent Good Fair Poor Cure rate (%) Mild 0 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0.0 Moderate (62.5) 8 (33.3) 1 (4.2) 0 (0.0) 95.8 Severe (38.2) 27 (49.1) 5 (9.1) 2 (3.6) 87.3 No (48.6) 30 (40.5) 6 (8.1) 2 (2.7) 89.2 Yes 5 0 (0/5) 5 (5/5) 0 (0/5) 0 (0/5) 5/5 No (45.1) 33 (46.5) 4 (5.6) 2 (2.8) 91.5 Yes 8 4 (50.0) 2 (25.0) 2 (25.0) 0 (0.0) 75.0 No (47.8) 29 (43.3) 4 (6.0) 2 (3.0) 91.0 Yes 12 4 (33.3) 6 (50.0) 2 (16.7) 0 (0.0) 83.3 Mild 0 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0.0 Moderate 6 0 (0.0) 6 (100.0) 0 (0.0) 0 (0.0) Severe 7 1 (14.3) 5 (71.4) 1 (14.3) 0 (0.0) 85.7 No 11 1 (9.1) 9 (81.8) 1 (9.1) 0 (0.0) 90.9 Yes 2 0 (0/2) 2 (2/2) 0 (0/2) 0 (0/2) 2/2 No 6 1 (16.7) 4 (66.7) 1 (16.7) 0 (0.0) 83.3 Yes 7 0 (0.0) 7 (100.0) 0 (0.0) 0 (0.0) No 6 1 (16.7) 4 (66.7) 1 (16.7) 0 (0.0) 83.3 Yes 7 0 (0.0) 7 (100.0) 0 (0.0) 0 (0.0) Table 8. Eradication by pathogen at end of treatment Strains (%) Causative organism Eradication Persistence Total S. pneumoniae 10 (83.3) 2 (16.7) 83.3 (10/12) H. influenzae 28 (87.5) 4 (12.5) 87.5 (28/32) M. catarrhalis 4 (4/4) 0 (0/4) 4/4 S. pyogenes 4 (4/4) 0 (0/4) 4/4 Total 46 (88.5) 6 (11.5) 88.5 (46/52) moniae S. pneumoniae μ H. influenzae μ μ μ μ μ μ μ H. influenzae μ S. pneumoniae H. influenzae S. pyogenes M. catarrhalis
9 Table 9. Adverse events (subjective symptoms/objective findings) Relationship All causality Drug-related System organ class and preferred term (MedDRA/J V.14.0) Events Patients (%) Events Patients (%) Ear and labyrinth disorders Ear pain 1 1 (0.9) Eye disorders Conjunctivitis 1 1 (0.9) Gastrointestinal disorder Loose bowel (13.0) (12.2) Mushy stool 9 9 (7.8) 7 7 (6.1) Stools watery 7 7 (6.1) 7 7 (6.1) Abdominal pain 1 1 (0.9) 1 1 (0.9) Constipation 1 1 (0.9) 1 1 (0.9) Vomiting 3 3 (2.6) 1 1 (0.9) General disorders and administration site conditions Pyrexia 2 2 (1.7) 1 1 (0.9) Infections and infestations sinusitis 1 1 (0.9) Bronchitis 2 2 (1.7) Gastroenteritis 6 6 (5.2) Influenza 1 1 (0.9) Molluscum contagiosum 1 1 (0.9) Nasopharyngitis 5 5 (4.3) Otitis media acute 1 1 (0.9) Parotitis 1 1 (0.9) Pharyngitis 4 4 (3.5) Injury, poisoning and procedural complications Contusion 1 1 (0.9) Respiratory, thoracic and mediastinal disorders Asthma 3 3 (2.6) 1 1 (0.9) Cough 1 1 (0.9) Epistaxis 1 1 (0.9) Upper respiratory tract inflammation 1 1 (0.9) Skin and subcutaneous tissue disorders Dermatitis contact 1 1 (0.9) 1 1 (0.9) Dermatitis diaper 1 1 (0.9) Dry skin 1 1 (0.9) Eczema 1 1 (0.9) Erythema 1 1 (0.9) Rash 3 3 (2.6) 2 2 (1.7) Urticaria 1 1 (0.9) 1 1 (0.9) Total (48.7) (31.3) Table 10. Incidence of diarrhea by age group Diarrhea Grade 3 years old or less (n 47) Over 3 years old (n 68) Total (n 115) Events Patients (%) Events Patients (%) Patients (%) Loose bowel Mild 7 7 (14.9) 7 7 (10.3) 14 (12.2) Mushy stool Mild 3 3 (6.4) 4 4 (5.9) 7 (6.1) Moderate 6 6 (12.8) 0 0 (0.0) 6 (5.2) Stools watery Severe 1 1 (2.1) 0 0 (0.0) 1 (0.9) Total (36.2) (16.2) 28 (24.3) S. pneumoniae g g μ H. influenzae g μ g μ μ S. pneumoniae g M. catarrhalis
10 Table 11. Adverse drug reactions (abnormal laboratory data changes) Preferred term (MedDRA/J V.14.0) No. of subjects Events Patients (%) Alanine aminotransferase increased (0.9) Aspartate aminotransferase increased (1.9) Blood potassium increased (0.9) White blood cell count decreased (0.9) White blood cell count increased (0.9) Platelet count increased (3.6) Total (6.2) Table 12. Concentrations of free carnitine in serum after CDTR-PI administration Concentrations of free carnitine in serum ( mol/l) (n 87) Before administration End of administration 5 10 days after last administration Mean S.D Range S. pneumoniae H. influenzae M. catarrhalis S. pyogenes H. influenzae H. influenzae S. pneumoniae H. influenzae μ μ μ ω ω ω σ μ t C μ
11 CDTR plasma concentration ( g/ml) observed simulated from population mean parameters Time (hr) Fig. 2. CDTR plasma concentration after oral CDTR-PI administration. T MIC (%) /16 4/6 5/6 4/7 1/ MIC ( g/ml) Eradication Persistence Fig. 4. Relationship between MIC and T MIC with bacteriological efficacy. T MIC (%) /18 6/6 4/5 6/9 1/ MIC ( g/ml) Excellent, Good Failure, Poor Fig. 3. Relationship between MIC and T MIC with clinical efficacy. t μ III S. pneumoniae H. influenzae S. pneumoniae H. influenzae S. pneumoniae H. influenzae M. catarrhalis S. pyogenes S. pneumoniae H. influenzae S. pneumoniae H. influenzae S. pneumoniae H. influenzae
12 Age (yr) Very easy to take Easy to take Table 13. Medication compliance Common Patients (%) Hard to take Unable to take Unknown Total Easy to take (%) (42.6) 20 (42.6) 5 (10.6) 2 (4.3) 0 (0.0) 0 (0.0) (43.5) 25 (54.3) 0 (0.0) 0 (0.0) 0 (0.0) 1 (2.2) (27.3) 16 (72.7) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) Total 46 (40.0) 61 (53.0) 5 (4.3) 2 (1.7) 0 (0.0) 1 (0.9) S. pneumoniae H. influenzae S. pneumoniae H. influenzae
13 S. pneumoniae H. influenzae Streptococcus pneumoniae Haemophilus influenzae
14 Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae
日本化学療法学会雑誌第65巻第4号
 Mycoplasma pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae Key words Mycoplasma pneumoniae Mycoplasma pneumoniae M. pneumoniae I M. pneumoniae M. pneumoniae M. pneumoniae
Mycoplasma pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae Key words Mycoplasma pneumoniae Mycoplasma pneumoniae M. pneumoniae I M. pneumoniae M. pneumoniae M. pneumoniae
日本化学療法学会雑誌第59巻第5号
 Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
Feb THE JAPANESE JOURNAL OF ANTIBIOTICS Tebipenem pivoxil 1 1, Meiji Seika 2 Meiji Seika G 3 Meiji Seika Tebipen
 Feb. 2016 THE JAPANESE JOURNAL OF ANTIBIOTICS 69 1 53 53 Tebipenem pivoxil 1 1, 3 2 2 1 1 Meiji Seika 2 Meiji Seika G 3 Meiji Seika 2015 12 15 Tebipenem pivoxil 10% 2010 4 2013 3 3,547 3,540 3,540 3,331
Feb. 2016 THE JAPANESE JOURNAL OF ANTIBIOTICS 69 1 53 53 Tebipenem pivoxil 1 1, 3 2 2 1 1 Meiji Seika 2 Meiji Seika G 3 Meiji Seika 2015 12 15 Tebipenem pivoxil 10% 2010 4 2013 3 3,547 3,540 3,540 3,331
日本化学療法学会雑誌第57巻第6号
 Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae S. pneumoniae β β H. influenzae S. pneumoniae H. influenzae M. catarrhalis Key words Otalgia Fever Item Crying,Iritation,
Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae S. pneumoniae β β H. influenzae S. pneumoniae H. influenzae M. catarrhalis Key words Otalgia Fever Item Crying,Iritation,
日本化学療法学会雑誌第56巻第S-1号
 Key words Streptococcus pneumoniae µ µ H F Cl H H N N H N F COOH O Fig.1. Sitafloxacinstructure. I γ S. pneumoniae Haemophilus influenzae Table1. Observationandtestschedule Observation/Tests Informedconsent
Key words Streptococcus pneumoniae µ µ H F Cl H H N N H N F COOH O Fig.1. Sitafloxacinstructure. I γ S. pneumoniae Haemophilus influenzae Table1. Observationandtestschedule Observation/Tests Informedconsent
日本化学療法学会雑誌第61巻第4号
 μ μ μ μ μ μ Key words I β μ Sex Age (years) Height (cm) Evaluation items Table1.Characteristics of patients 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) male 12 8 7 27 female 2 1
μ μ μ μ μ μ Key words I β μ Sex Age (years) Height (cm) Evaluation items Table1.Characteristics of patients 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) male 12 8 7 27 female 2 1
日本化学療法学会雑誌第56巻第1号
 β β β β β Streptococcus pneumoniaehaemophilus influenzaemoraxella catarrhalismycoplasma pneumoniaechlamydia pneumoniae β Key wordsβ mys nilc laci ngis Table. Assessmentschedule Parameters Patientcharacteristics
β β β β β Streptococcus pneumoniaehaemophilus influenzaemoraxella catarrhalismycoplasma pneumoniaechlamydia pneumoniae β Key wordsβ mys nilc laci ngis Table. Assessmentschedule Parameters Patientcharacteristics
THE JAPANESE JOURNAL OF ANTIBIOTICS 68 3 June 2015 Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis % 2 S. pneumon
 June 2015 THE JAPANESE JOURNAL OF ANTIBIOTICS 68 3 189 49 1 : 14 1 2 2 3 1 2 3 2015 4 3 1 : 14 CVA/AMPC 1 : 14 27 CVA/AMPC 1 : 14 88.5% Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis
June 2015 THE JAPANESE JOURNAL OF ANTIBIOTICS 68 3 189 49 1 : 14 1 2 2 3 1 2 3 2015 4 3 1 : 14 CVA/AMPC 1 : 14 27 CVA/AMPC 1 : 14 88.5% Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis
日本化学療法学会雑誌第57巻第4号
 Streptococcus pneumoniae Haemophilus influenzae β β Key words I β Enterococcus faecium Pseudomonas aeruginosa Streptococcus pneumoniae S. pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Table.
Streptococcus pneumoniae Haemophilus influenzae β β Key words I β Enterococcus faecium Pseudomonas aeruginosa Streptococcus pneumoniae S. pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Table.
208 ( 2 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 63 _ 3 June 2010 Cefditoren pivoxil (CDTR-PI) MS MS 10%
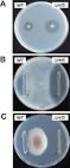 207 ( 1 ) Cefditoren pivoxil G 2010 2 2 2006 3 Cefditoren pivoxil CDTR-PI MS 10% CDTR-PI 305 2,144 2,006 1,958 1.79% 36 2,006 26 (1.30%) CDTR-PI CDTR-PI (9 mg/kg/day) 1.5 2 (2.70%) 2 (1.92%) 93.5% 1,831
207 ( 1 ) Cefditoren pivoxil G 2010 2 2 2006 3 Cefditoren pivoxil CDTR-PI MS 10% CDTR-PI 305 2,144 2,006 1,958 1.79% 36 2,006 26 (1.30%) CDTR-PI CDTR-PI (9 mg/kg/day) 1.5 2 (2.70%) 2 (1.92%) 93.5% 1,831
日本化学療法学会雑誌第55巻第S-1号
 µ µ Key words Streptococcus pneu- moniae S. pneumoniae µ µ I γ H H C HN F F O N O CO H H C SO H H O Fig.. ChemicalstructureofGRNX. γ γ II Haemophilus influenzae Pseudomonas aeruginosa Streptococcus intermedius
µ µ Key words Streptococcus pneu- moniae S. pneumoniae µ µ I γ H H C HN F F O N O CO H H C SO H H O Fig.. ChemicalstructureofGRNX. γ γ II Haemophilus influenzae Pseudomonas aeruginosa Streptococcus intermedius
日本化学療法学会雑誌第54巻第S-1号
 β β Key words β Candida krusei Candida glabrata β I β Drugadministration Baselinecharacteristics Clinicalsymptom Mycologicaltests Plasmadrugconcn Adverseevents Laboratorytests ) -leadecg Clinicalphotograph
β β Key words β Candida krusei Candida glabrata β I β Drugadministration Baselinecharacteristics Clinicalsymptom Mycologicaltests Plasmadrugconcn Adverseevents Laboratorytests ) -leadecg Clinicalphotograph
日本化学療法学会雑誌第61巻第6号
 β Moraxella catarrhalis Escherichia coli Citrobacter Klebsiella pneumoniae Enterobacter cloacae Serratia marcescens Proteus Pseudomonas aeruginosa Acinetobacter Bacteroides fragilis β Haemophilus influenzae
β Moraxella catarrhalis Escherichia coli Citrobacter Klebsiella pneumoniae Enterobacter cloacae Serratia marcescens Proteus Pseudomonas aeruginosa Acinetobacter Bacteroides fragilis β Haemophilus influenzae
CHEMOTHERAPY FEB Table 1. Activity of cefpirome and others against clinical isolates
 VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
CHEMOTHERAPY
 CHEMOTHERAPY VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin CHEMOTHERAPY AUG. 1993 VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin Table 1. Comparative in vitro activity of teicoplanin
CHEMOTHERAPY VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin CHEMOTHERAPY AUG. 1993 VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin Table 1. Comparative in vitro activity of teicoplanin
VOL. 40 S- 1 Table 1. Susceptibility of methicillin-resistant Staphylococcus aureus to meropenem Table 2. Coagulase typing of methicillin-resistant St
 CHEMOTHERAPY VOL. 40 S- 1 Table 1. Susceptibility of methicillin-resistant Staphylococcus aureus to meropenem Table 2. Coagulase typing of methicillin-resistant Staphylococcus aureus CHEMOTHERAPY Table
CHEMOTHERAPY VOL. 40 S- 1 Table 1. Susceptibility of methicillin-resistant Staphylococcus aureus to meropenem Table 2. Coagulase typing of methicillin-resistant Staphylococcus aureus CHEMOTHERAPY Table
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S.
 Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study
 CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
Fig. 1 Chemical structure of norfioxacin (AM-715)
 Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
CHEMOTHERAPY Fig. 1 Chemical structure of CXM-AX
 Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
CHEMOTHERAPY Table 1 Clinical effect of Sultamicillin
 CHEMOTHERAPY CHEMOTHERAPY Table 1 Clinical effect of Sultamicillin CHEMOTHERAPY Fig. 1 MICs of sultamicillin against respiratory pathogenic Branhamella catarrhalis 62 strains, inoculum size 106CFU/m1 Fig.
CHEMOTHERAPY CHEMOTHERAPY Table 1 Clinical effect of Sultamicillin CHEMOTHERAPY Fig. 1 MICs of sultamicillin against respiratory pathogenic Branhamella catarrhalis 62 strains, inoculum size 106CFU/m1 Fig.
日本化学療法学会雑誌第64巻第4号
 β β Moraxella catarrhalisescherichia colicitrobacter Klebsiella pneumoniaeenterobacter cloacaeserratia marcescens Proteus Providencia Pseudomonas aeruginosaacinetobacter Bacteroides fragilis β β E. colik.
β β Moraxella catarrhalisescherichia colicitrobacter Klebsiella pneumoniaeenterobacter cloacaeserratia marcescens Proteus Providencia Pseudomonas aeruginosaacinetobacter Bacteroides fragilis β β E. colik.
日本化学療法学会雑誌第50巻第6号
 13 11 30 14 4 22 Streptococcus pneumoniae Haemophilus influenzae amoxicillin AMPC cefditoren pivoxil CDTR AMPC 50 mg kg day CDTR 9mg kg day CDTR 18 mg kg day S. pneumoniae H. influenzae 8 17 25 1 25 S.
13 11 30 14 4 22 Streptococcus pneumoniae Haemophilus influenzae amoxicillin AMPC cefditoren pivoxil CDTR AMPC 50 mg kg day CDTR 9mg kg day CDTR 18 mg kg day S. pneumoniae H. influenzae 8 17 25 1 25 S.
日本化学療法学会雑誌第65巻第3号
 μ Key words Chlamydia trachomatis C. trachomatis I Table1.Observation items, categories and scores Observation item Category Score Body temperature () Lower abdominal pain Uterine corpus tenderness Condition
μ Key words Chlamydia trachomatis C. trachomatis I Table1.Observation items, categories and scores Observation item Category Score Body temperature () Lower abdominal pain Uterine corpus tenderness Condition
日本化学療法学会雑誌第58巻第4号
 Escherichia coli Enterococcus faecalisstreptococcus agalactiae Klebsiella pneumoniae Staphylococcus epidermidis E. colie. faecalispseudomonas aeruginosa K. pneumoniae S. agalactiae E. coli E. coli μ p
Escherichia coli Enterococcus faecalisstreptococcus agalactiae Klebsiella pneumoniae Staphylococcus epidermidis E. colie. faecalispseudomonas aeruginosa K. pneumoniae S. agalactiae E. coli E. coli μ p
180 ( 64 ) THE JAPANESE JOURNAL OF ANTIBIOTICS June 2011 A b group A Streptococcus: GAS GAS 10 meta-analysis 1) 2,3) GAS potential pathogens Tab
 June 2011 THE JAPANESE JOURNAL OF ANTIBIOTICS 64 23 179 ( 63 ) A b cefditoren pivoxil 5 amoxicillin 10 1,2) 1,3) 1) 1) 1) 4) 5) 6) 7) 1) 2) 3) 4) 5) 6) 7) 2011 4 8 2007 5 2009 4 A b GAS cefditoren pivoxil
June 2011 THE JAPANESE JOURNAL OF ANTIBIOTICS 64 23 179 ( 63 ) A b cefditoren pivoxil 5 amoxicillin 10 1,2) 1,3) 1) 1) 1) 4) 5) 6) 7) 1) 2) 3) 4) 5) 6) 7) 2011 4 8 2007 5 2009 4 A b GAS cefditoren pivoxil
Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone
 Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone (inoculum size= 106 CFU/ml) (Ĉ-lactamase producer : 2 strains) Fig. 1. Sensitivity distribution of
Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone (inoculum size= 106 CFU/ml) (Ĉ-lactamase producer : 2 strains) Fig. 1. Sensitivity distribution of
 172( 38 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 57 2 Apr. 2002 1 19 6 2002 5 8 4 254 254 (PBP) 90 83 65 142 PBP pbp1a, pbp2x, pbp2b 121 (49%), pbp1a, pbp2x 30 (12%), pbp2x, pbp2b 16 (6%), pbp2x 61 (24%),
172( 38 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 57 2 Apr. 2002 1 19 6 2002 5 8 4 254 254 (PBP) 90 83 65 142 PBP pbp1a, pbp2x, pbp2b 121 (49%), pbp1a, pbp2x 30 (12%), pbp2x, pbp2b 16 (6%), pbp2x 61 (24%),
CHEMOTHERAPY JUN Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter cloacae 27, Proteus rettgeri 7, Proteus inconstans 20, Proteus
 VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
Fig. 1 Chemical structure of DL-8280
 Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
日本化学療法学会雑誌第59巻第6号
 P P Streptococcus pneumoniae S. pneumoniae Key words Streptococcus pneumoniae Fig. 1. Capsular swelling reaction of S. pneumoniae type 6B. Cells are stained dark blue using a methylene blue. Capsule is
P P Streptococcus pneumoniae S. pneumoniae Key words Streptococcus pneumoniae Fig. 1. Capsular swelling reaction of S. pneumoniae type 6B. Cells are stained dark blue using a methylene blue. Capsule is
 CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates
 Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
CHEMOTHERAPY aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Prote
 aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Proteus mirabilis 3.13, Proteus vulgaris 1.56, Citrobacter freundii 0.39,
aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Proteus mirabilis 3.13, Proteus vulgaris 1.56, Citrobacter freundii 0.39,
Fig.2. Sensitivity distribution of clinical isolates of S. epidermidis (24 strains, 106 CFU/ml) Staphylococcus aureus Staphylococcus epider- midis Ent
 Fig.2. Sensitivity distribution of clinical isolates of S. epidermidis (24 strains, 106 CFU/ml) Staphylococcus aureus Staphylococcus epider- midis Enterococcus faecalis Klebsiella pneumoniae, Morganella
Fig.2. Sensitivity distribution of clinical isolates of S. epidermidis (24 strains, 106 CFU/ml) Staphylococcus aureus Staphylococcus epider- midis Enterococcus faecalis Klebsiella pneumoniae, Morganella
08-g-”O−}„j‹ê-4.02
 200( 96 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 58 2 Apr. 585 1 17 MIC 1. (Transtracheal aspiration: TTA) TTA TTA TTA 1400 TTA (diffuse panbronchiolitis, DPB) 1) erythromycin(em) EM 12.5% 87.5% 2,3) EM EM
200( 96 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 58 2 Apr. 585 1 17 MIC 1. (Transtracheal aspiration: TTA) TTA TTA TTA 1400 TTA (diffuse panbronchiolitis, DPB) 1) erythromycin(em) EM 12.5% 87.5% 2,3) EM EM
 Table 1 Sensitivity distribution of clinical isolates 1. Escherichia coli Inoculum size: 106cells/ml 2. Klebsiella pneumoniae 3. Enterobacter cloacae 4. Serratia marcescens Inoculum size: 106cells/nil
Table 1 Sensitivity distribution of clinical isolates 1. Escherichia coli Inoculum size: 106cells/ml 2. Klebsiella pneumoniae 3. Enterobacter cloacae 4. Serratia marcescens Inoculum size: 106cells/nil
366 12 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 Dec. 2012 1 8 DNA 2,3 16 12 20 171 2008 12 2010 11 2 3,558 4.44% 1.65% 1.17% 90% 9 Escherichia coli -
 Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 365 11 sita oxacin 1 1 1 1 1 1 2 2 3 3 1 1 1 2 3 2012 9 14 sita oxacin STFX 50 mg 10% 2008 1 2008 12 2010 11 2 STFX 1,452 91.4% 1,235/1,351 95.9% 466/486
Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 365 11 sita oxacin 1 1 1 1 1 1 2 2 3 3 1 1 1 2 3 2012 9 14 sita oxacin STFX 50 mg 10% 2008 1 2008 12 2010 11 2 STFX 1,452 91.4% 1,235/1,351 95.9% 466/486
988 CHEMOTHERAPY NOV. 1971
 988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
VOL.35 S-2 CHEMOTHERAPY Table 1 Sex and age distribution Table 2 Applications of treatment with carumonam Table 3 Concentration of carumonam in human
 CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
VOL.42 S-1
 CHEMOTHERAPY APR. 1994 VOL.42 S-1 CHEMOTHERAPY APR. 1994 Table 1. Criteria for evaluation of clinical efficacy by the Japanese Society of Oral and Maxillo-Facial Surgeons Grades of symptoms and numerical
CHEMOTHERAPY APR. 1994 VOL.42 S-1 CHEMOTHERAPY APR. 1994 Table 1. Criteria for evaluation of clinical efficacy by the Japanese Society of Oral and Maxillo-Facial Surgeons Grades of symptoms and numerical
日本化学療法学会雑誌第57巻第5号
 in vitro Key words Streptococcus pneumoniae β Haemophilus influenzae Escherichia coli I Table. Antibacteriallevofloxacinactivityagainstclinicalisolates Organism Year Susceptibility(%)(Numberofstrains)
in vitro Key words Streptococcus pneumoniae β Haemophilus influenzae Escherichia coli I Table. Antibacteriallevofloxacinactivityagainstclinicalisolates Organism Year Susceptibility(%)(Numberofstrains)
pneumoniae 30, C. freundii 32, E. aerogenes 27, E. cloacae 32, P. mirabilis 31, P. vulgaris 34, M. morganii 32, S. marcescens 31, H. influenzae 27, P.
 pneumoniae 30, C. freundii 32, E. aerogenes 27, E. cloacae 32, P. mirabilis 31, P. vulgaris 34, M. morganii 32, S. marcescens 31, H. influenzae 27, P. aeruginosa 30, P. maltophilia pyogenes 32, Escherichia
pneumoniae 30, C. freundii 32, E. aerogenes 27, E. cloacae 32, P. mirabilis 31, P. vulgaris 34, M. morganii 32, S. marcescens 31, H. influenzae 27, P. aeruginosa 30, P. maltophilia pyogenes 32, Escherichia
VOL. 34 S-2 CHEMOTH8RAPY 913
 VOL. 34 S-2 CHEMOTH8RAPY 913 914 CHEMOTHERAPY APR. 1986 Fig. 1 Chemical structure of T-2588 and T-2525 T- 2588 pivaloyloxymethyl (+ )- (6 R, 7 R)-7-[(Z)-2- (2-amino- 4-thiazolyl)-2-methox yiminoacetamido]-3-[(
VOL. 34 S-2 CHEMOTH8RAPY 913 914 CHEMOTHERAPY APR. 1986 Fig. 1 Chemical structure of T-2588 and T-2525 T- 2588 pivaloyloxymethyl (+ )- (6 R, 7 R)-7-[(Z)-2- (2-amino- 4-thiazolyl)-2-methox yiminoacetamido]-3-[(
 600mg 600mg CTD 2 2.5 2.5 Page 3 2.5...7 2.5.1...7 2.5.2...27 2.5.3...28 2.5.4...42 2.5.5...55 2.5.6...79 2.5.7...97 2.5 Page 5 73 67 31 48 48A 102 104 105 106 ALP ALT(GPT) AST(GOT) AUC AUEC BID BUN
600mg 600mg CTD 2 2.5 2.5 Page 3 2.5...7 2.5.1...7 2.5.2...27 2.5.3...28 2.5.4...42 2.5.5...55 2.5.6...79 2.5.7...97 2.5 Page 5 73 67 31 48 48A 102 104 105 106 ALP ALT(GPT) AST(GOT) AUC AUEC BID BUN
CHEMOTHERAPY
 CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of Sulbactam/CPZ against standard strains MIC mg/ml Inoculum size 106 CFU/ml * Sulbactam/CPZ= 1: 1 ** Concentration of Sulbactam+ CPZ CHEMOTHERAPY
CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of Sulbactam/CPZ against standard strains MIC mg/ml Inoculum size 106 CFU/ml * Sulbactam/CPZ= 1: 1 ** Concentration of Sulbactam+ CPZ CHEMOTHERAPY
coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, F
 VOL.43 S-1 coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, Flavobacter- Table 1. Concentration of grepafloxacin
VOL.43 S-1 coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, Flavobacter- Table 1. Concentration of grepafloxacin
VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity of gram nega
 1066 CHEMOTHERAPY MAR. 1975 Table 1 Sensitivity of standard strains VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity
1066 CHEMOTHERAPY MAR. 1975 Table 1 Sensitivity of standard strains VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity
 1) Chemical name: Fig. 1 Chemical structure of TE-031 (-)-(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2, PYranosyl)oxy]-14-ethyl-12,13-dihydroxy-7-meth 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexo- oxy-3,5,7,9,11,13-hexamethy1-6-[[3,4,6-trideoxy-3-
1) Chemical name: Fig. 1 Chemical structure of TE-031 (-)-(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2, PYranosyl)oxy]-14-ethyl-12,13-dihydroxy-7-meth 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexo- oxy-3,5,7,9,11,13-hexamethy1-6-[[3,4,6-trideoxy-3-
日本化学療法学会雑誌第56巻第3号
 β Key words Candida albicans albicans Candida C. albicans Candida glabrata Candida krusei albicans Candida C. glabrata C. albicans albicans Candida β in vitro in vivo C. glabrata C. krusei I β γ µ Candida
β Key words Candida albicans albicans Candida C. albicans Candida glabrata Candida krusei albicans Candida C. glabrata C. albicans albicans Candida β in vitro in vivo C. glabrata C. krusei I β γ µ Candida
日本化学療法学会雑誌第51巻第2号
 piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
 Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
_02三浦.indd
 36, 7-11, 2016 IPD PCV 5 3 80% 1 invasive pneumococcal disease ; IPD 2010 7 PCV7 2013 13 PCV13 IPD IPD 2,3 2015 1 2015 12 5 1 IPD 2015 1 12 IPD 5 IPD 1 4 2 5 http://pneumocatch.jp/ 5 Statens Serum Institut
36, 7-11, 2016 IPD PCV 5 3 80% 1 invasive pneumococcal disease ; IPD 2010 7 PCV7 2013 13 PCV13 IPD IPD 2,3 2015 1 2015 12 5 1 IPD 2015 1 12 IPD 5 IPD 1 4 2 5 http://pneumocatch.jp/ 5 Statens Serum Institut
日本化学療法学会雑誌第58巻第2号
 Key words β Candida Table1. MCFGCPAstudygroup Investigator (representative) YoshitsuguMiyazaki NaoyukiMiyashita RyoichiAmitani KenjiOgawa AtsuyukiKurashima ToshiroKiguchi MichiakiMishima YuichiInoue HiroshiSaito
Key words β Candida Table1. MCFGCPAstudygroup Investigator (representative) YoshitsuguMiyazaki NaoyukiMiyashita RyoichiAmitani KenjiOgawa AtsuyukiKurashima ToshiroKiguchi MichiakiMishima YuichiInoue HiroshiSaito
DIC vegetation 1 nonbacterial thrombogenic e
 2001 2002 Guidelines for the Prevention and Treatment of Infective Endocarditis (JCS 2003) h 1 1 2 3 4 5 6 7 8 9 2 1 2 3 1 2 3 4 1 2 5 1 2 1 G Streptococcus viridans Streptococcus bovis 2 G Streptococcus
2001 2002 Guidelines for the Prevention and Treatment of Infective Endocarditis (JCS 2003) h 1 1 2 3 4 5 6 7 8 9 2 1 2 3 1 2 3 4 1 2 5 1 2 1 G Streptococcus viridans Streptococcus bovis 2 G Streptococcus
 CHEMOTHERAPY APRIL 1992 VOL. 40 S- 1 Table 1-1. Comparative in vitro activity of meropenem against clinical isolates CNS: coagulase-negative staphylococci CHEMOTHERAPY APRIL 1992 Table 1-2. Comparative
CHEMOTHERAPY APRIL 1992 VOL. 40 S- 1 Table 1-1. Comparative in vitro activity of meropenem against clinical isolates CNS: coagulase-negative staphylococci CHEMOTHERAPY APRIL 1992 Table 1-2. Comparative
日本化学療法学会雑誌第54巻第1号
 Chlamydophila pneumoniae Chlamydophila psittaci Mycoplasma pneumoniae Legionella pneumophila C. pneumoniae C. psittaci C. pneumoniae C. psittaci M. pneumoniae M. pneumoniae C. psittaci L. pneumophila C.
Chlamydophila pneumoniae Chlamydophila psittaci Mycoplasma pneumoniae Legionella pneumophila C. pneumoniae C. psittaci C. pneumoniae C. psittaci M. pneumoniae M. pneumoniae C. psittaci L. pneumophila C.
 Table 1.Concentration of gatifloxacin (Middle-ear) Table 2.Concentration of gatifloxacin (Paranasal sinuses) Table 3.Concentration of gatifloxacin (Tonsil) Table 4.No.of patients studied Table 5.Background
Table 1.Concentration of gatifloxacin (Middle-ear) Table 2.Concentration of gatifloxacin (Paranasal sinuses) Table 3.Concentration of gatifloxacin (Tonsil) Table 4.No.of patients studied Table 5.Background
Table 1 Classification of female patients with vesical irritating symptom by their signs : Urinary pain with or without other vesical irritability. s
 Table 1 Classification of female patients with vesical irritating symptom by their signs : Urinary pain with or without other vesical irritability. s Vesical irritability without urinary Pain. Pyuria 10/
Table 1 Classification of female patients with vesical irritating symptom by their signs : Urinary pain with or without other vesical irritability. s Vesical irritability without urinary Pain. Pyuria 10/
Fig. 1 Chemical structure of KW-1070
 Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels of mezlocillin
 CHEMOTHERAPY Fig. 2 Urinary excretion of mezlocillin Fig. 1 Blood levels of mezlocillin CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels
CHEMOTHERAPY Fig. 2 Urinary excretion of mezlocillin Fig. 1 Blood levels of mezlocillin CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels
Staphylococcus sp. K.pneumoniae P.mirabilis C.freundii E. cloacae Serratia sp. P. aeruginosa ml, Enterococcus avium >100ƒÊg/ml
 CHEMOTHERAPY SEPT. 1992 cefoperazone ceftazidime (CAZ), imipenem (IPM) Staphylococcus sp., Enterococcus (CPZ), faecalis, Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii, Enterobacter cloacae,
CHEMOTHERAPY SEPT. 1992 cefoperazone ceftazidime (CAZ), imipenem (IPM) Staphylococcus sp., Enterococcus (CPZ), faecalis, Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii, Enterobacter cloacae,
CHEMOTHERAPY Methicillin-resistant S.aureus(MRSA) coccus epidermidis 105 Streptococcus pyogenes E.faecali senterococcus avium Enterococcus faecium Str
 cefaclor(ccl),cefuroxime(cxm),cefixime (CFIX),cefteram(CFTM),cefdinir(CFDN) pneumoniae,streptococcus pyogenes Moraxella catarrhalis,haemophilus influenzae,escherichia coli, Klebsiella pneumoniae,proteus
cefaclor(ccl),cefuroxime(cxm),cefixime (CFIX),cefteram(CFTM),cefdinir(CFDN) pneumoniae,streptococcus pyogenes Moraxella catarrhalis,haemophilus influenzae,escherichia coli, Klebsiella pneumoniae,proteus
日本化学療法学会雑誌第57巻第S-2号
 Key words β I Table. Observationandtestschedule Testschedule Observation/Tests Beforetreatment Endoftreatment 5 9days aftercompletion oftreatment 4 6weeks aftercompletion oftreatment Informedconsent Patientbackground
Key words β I Table. Observationandtestschedule Testschedule Observation/Tests Beforetreatment Endoftreatment 5 9days aftercompletion oftreatment 4 6weeks aftercompletion oftreatment Informedconsent Patientbackground
400 46 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 Dec. 2012 LVFX 100 mg 3 / 7 150 mg 2 / 7 2 2006 2008 9 LVFX PK PD 2009 7 100 mg 1 3 500 mg 1 1 AUC/MIC
 Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
CHEMOTHERAPY NOV S. aureus, S. epidermidis, E. coli, K. pgeumoniae, E. cloacae, S. marcescens, P. mirabilis, Proteus, P. aeruginosa Inoculum siz
 VOL.33 S-5 CHEMOTHERAPY 381 Fig. 1 Chemical structure of HAPA-B Chemical name 1-N-[(2S)-3-Amino-2-hydroxypropiony1]-4-0-(6-amino- 6-deoxy-a-D-glucopyranosyl)-6-013-deoxy-4-C-methyl- 3-(methylamino)-ƒÀ-L-arabinopyranosyl]-2-deoxystreptamine
VOL.33 S-5 CHEMOTHERAPY 381 Fig. 1 Chemical structure of HAPA-B Chemical name 1-N-[(2S)-3-Amino-2-hydroxypropiony1]-4-0-(6-amino- 6-deoxy-a-D-glucopyranosyl)-6-013-deoxy-4-C-methyl- 3-(methylamino)-ƒÀ-L-arabinopyranosyl]-2-deoxystreptamine
 Table 1. Antibacterial activity of cefdinir, cefixime, cefteram, cefuroxime, cefaclor and amoxicillin against standard strains Inoculum size: 108 cells/ml CFDN: cefdinir, CFIX: cefixime, CFTM: cefteram,
Table 1. Antibacterial activity of cefdinir, cefixime, cefteram, cefuroxime, cefaclor and amoxicillin against standard strains Inoculum size: 108 cells/ml CFDN: cefdinir, CFIX: cefixime, CFTM: cefteram,
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST
 Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
 Table 1 Classification of female patients with vealcal irritating symptom by their signs Urination pain with other vesical irritability or not Table 2 Serum levels of DL-8280 after a single oral administration
Table 1 Classification of female patients with vealcal irritating symptom by their signs Urination pain with other vesical irritability or not Table 2 Serum levels of DL-8280 after a single oral administration
Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution of female patients with blad
 Key words: Female with bladder irritability, Subjective symptoms, Pyuria, Bacteriuria Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution
Key words: Female with bladder irritability, Subjective symptoms, Pyuria, Bacteriuria Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution
CHEMOTHERAPY
 CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of BRL 28500 against standard strains of bacteria Fig, 1 Sensitivity distribution of ABPC-resistant E. coli isolated from urinary tract Fig. 2 Sensitivity
CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of BRL 28500 against standard strains of bacteria Fig, 1 Sensitivity distribution of ABPC-resistant E. coli isolated from urinary tract Fig. 2 Sensitivity
VOL.42 S-1 methicillin-susceptible Staphylococcus aureus (MSSA), Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Klebsiella pneum
 VOL.42 S-1 methicillin-susceptible Staphylococcus aureus (MSSA), Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae methicillin-resistant Staphylococcus
VOL.42 S-1 methicillin-susceptible Staphylococcus aureus (MSSA), Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae methicillin-resistant Staphylococcus
2 2 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 1 Feb. 2012 CLSI M100-S17benzylpenicillin PCG MIC 0.05 g/ml S. pneumoniae penicillin-susceptible S. pneumon
 Feb. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 1 1 1 2008 2009 2011 10 25 2008 6 2009 4 Streptococcus pneumoniae 377 penicillin-binding protein: PBP 2004 S. pneumoniae 160 2 2 THE JAPANESE JOURNAL OF
Feb. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 1 1 1 2008 2009 2011 10 25 2008 6 2009 4 Streptococcus pneumoniae 377 penicillin-binding protein: PBP 2004 S. pneumoniae 160 2 2 THE JAPANESE JOURNAL OF
1272 CHEMOTHERAPY MAR. 1975
 1272 CHEMOTHERAPY MAR. 1975 VOL. 23 NO. 3 CHEMOTHERAPY 1273 Fig. 2 Minimal inhibitory concentration of aminoglycosides against 50 strains of Klebsiella Fig. 1 Minimal inhibitory concentration of aminoglycosides
1272 CHEMOTHERAPY MAR. 1975 VOL. 23 NO. 3 CHEMOTHERAPY 1273 Fig. 2 Minimal inhibitory concentration of aminoglycosides against 50 strains of Klebsiella Fig. 1 Minimal inhibitory concentration of aminoglycosides
VOL.32 S-7 CHEMOTHERAPY Table 1 MIC of standard strains of CTRX Fig. 2 Cumulative curves of MIC S. aureus (26 strains )
 CHEMOTHERAPY OCT. 1984 Fig. I Chemical structure of CTRX VOL.32 S-7 CHEMOTHERAPY Table 1 MIC of standard strains of CTRX Fig. 2 Cumulative curves of MIC S. aureus (26 strains ) CHEMOTHERAPY Fig. 3 Cumulative
CHEMOTHERAPY OCT. 1984 Fig. I Chemical structure of CTRX VOL.32 S-7 CHEMOTHERAPY Table 1 MIC of standard strains of CTRX Fig. 2 Cumulative curves of MIC S. aureus (26 strains ) CHEMOTHERAPY Fig. 3 Cumulative
CHEMOTHERAPY MAY. 1988
 CHEMOTHERAPY MAY. 1988 CHEMOTHERAPY Fig. 1 Chemical structure CHEMOTHERAPY MAY. 1988 VOL.36 5-1 CHEMOTHERAPY CHEMOTHERAPY MAY. 1988 VOL.36 S-1 CHEMOTHERAPY CHEMOTHERAPY MAY. 1988 VOL.36 S-1 CHEMOTHERAPY
CHEMOTHERAPY MAY. 1988 CHEMOTHERAPY Fig. 1 Chemical structure CHEMOTHERAPY MAY. 1988 VOL.36 5-1 CHEMOTHERAPY CHEMOTHERAPY MAY. 1988 VOL.36 S-1 CHEMOTHERAPY CHEMOTHERAPY MAY. 1988 VOL.36 S-1 CHEMOTHERAPY
 CHEMOTHERAPY Silver sulfadiazine (T 107) CHEMOTHERAPY Fig. 1 Item of patients Table 1 Criteria of bacteriological efficacy by the Committee xcellent: E Score of infection becomes to 0% at the end of medication.
CHEMOTHERAPY Silver sulfadiazine (T 107) CHEMOTHERAPY Fig. 1 Item of patients Table 1 Criteria of bacteriological efficacy by the Committee xcellent: E Score of infection becomes to 0% at the end of medication.
CHEMOTHERAPY DEC phvlococcus aureus Staphylococcus Enterococcus faecalis Escherichia Klebsiella pneumoniae Serratia marcescens Pseudomonas cepac
 CHEMOTHERAPY DEC. 1988 phvlococcus aureus Staphylococcus Enterococcus faecalis Escherichia Klebsiella pneumoniae Serratia marcescens Pseudomonas cepacia 1 Bacteroides bivius Propionibacterium granulosum
CHEMOTHERAPY DEC. 1988 phvlococcus aureus Staphylococcus Enterococcus faecalis Escherichia Klebsiella pneumoniae Serratia marcescens Pseudomonas cepacia 1 Bacteroides bivius Propionibacterium granulosum
 The clinical characteristics of Mycoplasma pneumoniae pneumonia in children younger than 6 years old Nobue Takeda1,2),Tomomichi Kurosaki1),Naruhiko Ishiwada2),Yoichi Kohno2) 1)Department of Pediatrics,Chiba
The clinical characteristics of Mycoplasma pneumoniae pneumonia in children younger than 6 years old Nobue Takeda1,2),Tomomichi Kurosaki1),Naruhiko Ishiwada2),Yoichi Kohno2) 1)Department of Pediatrics,Chiba
 VOL.32 S-9 CHEMOTHERAPY Table 1 Minimum inhibitory concentrations of AC-1370, CPZ and CAZ Table 2 Efficacy of AC-1370 and CPZ against systemic infections in mice *Inoculum size: 106 cells/ml * 95% confidence
VOL.32 S-9 CHEMOTHERAPY Table 1 Minimum inhibitory concentrations of AC-1370, CPZ and CAZ Table 2 Efficacy of AC-1370 and CPZ against systemic infections in mice *Inoculum size: 106 cells/ml * 95% confidence
Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-
 Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl) oxy]-14-ethyl-12,
Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl) oxy]-14-ethyl-12,
小児感染症分離株における感受性サーベイランス
 小児感染症分離株における 感受性サーベイランス 公益社団法人日本化学療法学会, 一般社団法人日本感染症学会, 日本小児感染症学会小児用キノロン薬適正使用推進委員会委員長 : 渡辺彰委員 : 岩田敏, 坂田宏, 佐藤吉壮, 鈴木賢二, 宮下修行, 堀誠治, 山口禎夫協力委員 : 小田島正明, 交久瀬善隆, 長谷川寿一, 牧展子, 和田光市 はじめに 2009 年 12 月に耐性菌感染症治療のために二次選択薬として承認された小児用キノロン薬の再審査期間の
小児感染症分離株における 感受性サーベイランス 公益社団法人日本化学療法学会, 一般社団法人日本感染症学会, 日本小児感染症学会小児用キノロン薬適正使用推進委員会委員長 : 渡辺彰委員 : 岩田敏, 坂田宏, 佐藤吉壮, 鈴木賢二, 宮下修行, 堀誠治, 山口禎夫協力委員 : 小田島正明, 交久瀬善隆, 長谷川寿一, 牧展子, 和田光市 はじめに 2009 年 12 月に耐性菌感染症治療のために二次選択薬として承認された小児用キノロン薬の再審査期間の
日本化学療法学会雑誌第57巻第1号
 In vitro Streptococcus pneumoniae Escherichia coli in vitro Streptococcus pneumoniae Escherichia coli µs. pneumoniae E. coli µ Key words in vitrostreptococcus pneumoniae Escherichia coli Escherichia coli
In vitro Streptococcus pneumoniae Escherichia coli in vitro Streptococcus pneumoniae Escherichia coli µs. pneumoniae E. coli µ Key words in vitrostreptococcus pneumoniae Escherichia coli Escherichia coli
 Table 1. Influence of urine ph on MBCs of new quinolones against Escherichia coli NIHJ JC-2 and Pseudomonas aeruginosa 18S; MBCs in urine were compared with those in Miieller-Hinton broth. Table 2. Influence
Table 1. Influence of urine ph on MBCs of new quinolones against Escherichia coli NIHJ JC-2 and Pseudomonas aeruginosa 18S; MBCs in urine were compared with those in Miieller-Hinton broth. Table 2. Influence
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, Sc
 A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
Key words: Antibodies to Leptospira, Tokyo, Uveitis
 Key words: Antibodies to Leptospira, Tokyo, Uveitis Fig. 1 Distribution of Antibody Titers in Age Decade Fig. 3 Distribution of Antibody Titers in each Strain Fig. 2 Correlation between Antibody Titers
Key words: Antibodies to Leptospira, Tokyo, Uveitis Fig. 1 Distribution of Antibody Titers in Age Decade Fig. 3 Distribution of Antibody Titers in each Strain Fig. 2 Correlation between Antibody Titers
VOL.30 S-1 CHEMOTHERAPY Table 1 Antibacterial activity of CTT against standard strains Table 2 Antibacterial activity of CTT against standard strains
 CHEMOTHERAPY APR. 1982 Fig. 1 Chemical structure of cefotetan (CTT, YM09330) VOL.30 S-1 CHEMOTHERAPY Table 1 Antibacterial activity of CTT against standard strains Table 2 Antibacterial activity of CTT
CHEMOTHERAPY APR. 1982 Fig. 1 Chemical structure of cefotetan (CTT, YM09330) VOL.30 S-1 CHEMOTHERAPY Table 1 Antibacterial activity of CTT against standard strains Table 2 Antibacterial activity of CTT
3) ベースラインからの個々の変化量 付録 2.7.4:30 主な臨床検査値の推移 (045 試験 )( 続き ) Individual Change From Baseline in Creatinine (mg/dl) Over Time (All Patients as Treated) 24
 3) ベースラインからの個々の変化量 付録 2.7.4:30 主な臨床検査値の推移 (045 試験 )( 続き ) Individual Change From Baseline in Neutrophils (/microl) Over Time (All Patients as Treated) 24-wk MK-7009 300 mg b.i.d. + Peg-IFN + 10000 5000
3) ベースラインからの個々の変化量 付録 2.7.4:30 主な臨床検査値の推移 (045 試験 )( 続き ) Individual Change From Baseline in Neutrophils (/microl) Over Time (All Patients as Treated) 24-wk MK-7009 300 mg b.i.d. + Peg-IFN + 10000 5000
CHEMOTHERAPY DEC (NFLX), ofloxacin (OFLX), ciprofloxacin (CPFX) Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecali
 CHEMOTHERAPY DEC. 1988 (NFLX), ofloxacin (OFLX), ciprofloxacin (CPFX) Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis Pseudomonas aeruginosa, Serratia ma- Fig. 1. Chemical
CHEMOTHERAPY DEC. 1988 (NFLX), ofloxacin (OFLX), ciprofloxacin (CPFX) Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis Pseudomonas aeruginosa, Serratia ma- Fig. 1. Chemical
VOL.27S-1 CHEMOTHERAPY 109 Klebsiella, Proteus, Pseudomonas Streptococcus Fig. 1 Concentration in blood and in CSF after intravenous drip infusion of
 VOL.27S-1 CHEMOTHERAPY 109 Klebsiella, Proteus, Pseudomonas Streptococcus Fig. 1 Concentration in blood and in CSF after intravenous drip infusion of 4.9 g Mezlocillin Streptococcus pneumonzae pneumoniae
VOL.27S-1 CHEMOTHERAPY 109 Klebsiella, Proteus, Pseudomonas Streptococcus Fig. 1 Concentration in blood and in CSF after intravenous drip infusion of 4.9 g Mezlocillin Streptococcus pneumonzae pneumoniae
Fig.1 Chemical structure of BAY o 9867
 Fig.1 Chemical structure of BAY o 9867 CHEMOTHERAPY 43 Table 3 Antibacterial spectrum of gram negative bacteria Medium:Heart infusion agar (Nissui) Method:Agar dilution (Streak) CHEMOTHERAPY DEC 1985
Fig.1 Chemical structure of BAY o 9867 CHEMOTHERAPY 43 Table 3 Antibacterial spectrum of gram negative bacteria Medium:Heart infusion agar (Nissui) Method:Agar dilution (Streak) CHEMOTHERAPY DEC 1985
感染症学雑誌第77巻第8号
 1 2 µ Streptococcus pneumoniae µ S. pneumoniae µ S. pneumoniae pbp β β pbp1a pbp2x pbp2b pbp mefe ermb pbp pbp1a pbp2x pbp2b pbp pbp pbp pbp 2x pbp1a pbp2x pbp 2b mefe ermb pbp β pbp2x β β β µ µ pbp3 µ
1 2 µ Streptococcus pneumoniae µ S. pneumoniae µ S. pneumoniae pbp β β pbp1a pbp2x pbp2b pbp mefe ermb pbp pbp1a pbp2x pbp2b pbp pbp pbp pbp 2x pbp1a pbp2x pbp 2b mefe ermb pbp β pbp2x β β β µ µ pbp3 µ
日本化学療法学会雑誌第54巻第S-1号
 β β β Key words β β I HP- -CD CHR RHC R R R R CHR R R R R RHC R R R CHR R R R RHC CHR R H CH CH Fig.. Structuralformulaofhydroxypropyl-β -cyclodextrin(hp-β -CD). CH n H β β β β Table-. Dosageandadministration(Singleadministrationstudy)
β β β Key words β β I HP- -CD CHR RHC R R R R CHR R R R R RHC R R R CHR R R R RHC CHR R H CH CH Fig.. Structuralformulaofhydroxypropyl-β -cyclodextrin(hp-β -CD). CH n H β β β β Table-. Dosageandadministration(Singleadministrationstudy)
 Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
 VOL. 17 NO. 7 CHEMOTHERAPY 1305 1) W. BRumFirr et al. : Clinical and laboratory studies with carbenicillin. Lancet 1: 1289~ 1293, 1967 2) E. T. KNUDSEN et al. : A new semisynthetic penicillin active against
VOL. 17 NO. 7 CHEMOTHERAPY 1305 1) W. BRumFirr et al. : Clinical and laboratory studies with carbenicillin. Lancet 1: 1289~ 1293, 1967 2) E. T. KNUDSEN et al. : A new semisynthetic penicillin active against
VOL. 20 NO. 5 CHEMOTHERAPY Methoxy-4-sulfanilamidopyrimidine (OS-3376) Sulfadimethoxine (SDM) Table 1. In vitro antibacterial activities of OS-3
 VOL. 20 NO. 5 CHEMOTHERAPY 653 2-Methoxy-4-sulfanilamidopyrimidine (OS-3376) Sulfadimethoxine (SDM) Table 1. In vitro antibacterial activities of OS-3376, SDM, SIZ and SMZ against Gram-positive and negative
VOL. 20 NO. 5 CHEMOTHERAPY 653 2-Methoxy-4-sulfanilamidopyrimidine (OS-3376) Sulfadimethoxine (SDM) Table 1. In vitro antibacterial activities of OS-3376, SDM, SIZ and SMZ against Gram-positive and negative
cover
 日本耳鼻咽喉科感染症研究会会誌 (2012.05) 30 巻 1 号 :133~136. 急性中耳炎 急性鼻副鼻腔炎における抗菌薬の選択基準とバリアンス 林達哉 第 41 回日本耳鼻咽喉科感染症研究会 シンポジウム 上気道感染症治療の最前線 タイトル名 : 急性中耳炎 急性鼻副鼻腔炎における抗菌薬の選択基準とバリアンス 著者名 : 林達哉 ( はやしたつや ) 所属 : 旭川医科大学耳鼻咽喉科 頭頸部外科
日本耳鼻咽喉科感染症研究会会誌 (2012.05) 30 巻 1 号 :133~136. 急性中耳炎 急性鼻副鼻腔炎における抗菌薬の選択基準とバリアンス 林達哉 第 41 回日本耳鼻咽喉科感染症研究会 シンポジウム 上気道感染症治療の最前線 タイトル名 : 急性中耳炎 急性鼻副鼻腔炎における抗菌薬の選択基準とバリアンス 著者名 : 林達哉 ( はやしたつや ) 所属 : 旭川医科大学耳鼻咽喉科 頭頸部外科
Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII (45)
 Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2305(45) 2306(46) THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 Dec. Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2307(47) 2308(48) THE JAPANESE
Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2305(45) 2306(46) THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 Dec. Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2307(47) 2308(48) THE JAPANESE
CHEMOTHERAPY APR Fig. 2 The inactivation of aminoglycoside antibiotics by PC-904 Fig. 3 Serum concentration of PC-904 (1) Fig. 4 Urinary recover
 VOL.26 S-2 CHEMOTHERAPY Gentamicin (GM), Dibekacin (DKB), Tobramycin Fig. 1 Protein concentration and protein binding rate Table 2 Protein binding rate of PC-904 in serum of healthy adults, and patients
VOL.26 S-2 CHEMOTHERAPY Gentamicin (GM), Dibekacin (DKB), Tobramycin Fig. 1 Protein concentration and protein binding rate Table 2 Protein binding rate of PC-904 in serum of healthy adults, and patients
 Table 1 Survival rates of infected mice given antibiotic doses producing peak serum a) S. aurcus Smith Challenge dose :7 ~10 (5% mucin) CFU/mouse. LD50: 1 ~103 (5% mucin) CFU/mouse. Table 2 Survival rates
Table 1 Survival rates of infected mice given antibiotic doses producing peak serum a) S. aurcus Smith Challenge dose :7 ~10 (5% mucin) CFU/mouse. LD50: 1 ~103 (5% mucin) CFU/mouse. Table 2 Survival rates
