日本化学療法学会雑誌第61巻第4号
|
|
|
- あつとし うえや
- 7 years ago
- Views:
Transcription
1 μ μ μ μ μ μ Key words
2 I β μ
3 Sex Age (years) Height (cm) Evaluation items Table1.Characteristics of patients 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) male female Mean S.D Mean S.D Weight (kg) Mean S.D Diagnosis Invasive Pulmonary aspergillosis Pulmonary aspergilloma Candidemia 1 1 Disseminated candidiasis 1 1 Pulmonary cryptococcosis 3 3 Cryptococcal meningitis 1 1 Possible mycosis Severity of infection Underlying disease Antifungal pretreatment mild moderate severe yes no 1 1 yes no II
4 concentration (g/ml) concentration (g/ml) concentration (g/ml) (A) n (B) n (C) n Fig.1.Serum concentrations of amphotericin B after intravenous infusion of L-AMB at doses of 1. mg/kg (A), 2.5 mg/kg (B), or 5. mg/kg (C). μ μ μ μ μ μ μ β
5 Table2.Pharmacokinetic parameters of L-AMB in patients after intravenous infusion on the first day Dosage (mg/kg) 1. (n13) 2.5 (n9) 5. (n9) Cmax (g/ml) T1/2 (hr) AUC24 (ghr/ml) AUC (ghr/ml) Cltot (L/kg/hr) Vd (ss) (L/kg) Mean S.D Mean S.D Mean S.D Cmax (g/ml) AUC24 (g/ml) Dose (mg/kg) Dose (mg/kg) Fig.2.Dose dependence of L-AMB pharmacokinetics (Cmax or AUC: MeanS.D.). N13, 9 and 9 at doses of 1. mg/kg, 2.5 mg/kg and 5. mg/kg, respectively. III μ μ μ μ μ μ
6 concentration (g/ml) concentration (g/ml) concentration (g/ml) (A) n (B) n (C) n9 Fig.3.Serum trough concentrations of amphotericin B after intravenous infusion of L-AMB at doses of 1. mg/kg (A), 2.5 mg/kg (B), or 5. mg/kg (C). : Patients with increased serum trough concentrations of amphotericin B β
7 MedDRA SOC NAME MedDRA PT NAME Table3.Adverse drug reactions 1. mg/kg/day n 2.5 mg/kg/day n 5. mg/kg/day n Total n Total number of patients 1 (71.4%) 14 8 (88.9%) 9 9 (1%) 9 27 (84.4%) 32 Gastrointestinal disorders 4 (28.6%) 14 3 (33.3%) 9 6 (66.7%) 9 13 (4.6%) 32 Diarrhoea 2 (14.3%) 14 1 (11.1%) 9 2 (22.2%) 9 5 (15.6%) 32 Nausea 1 (7.1%) 14 1 (11.1%) 9 6 (66.7%) 9 8 (25.%) 32 Vomiting (%) 14 (%) 9 2 (22.2%) 9 2 (6.3%) 32 Constipation (%) 14 1 (11.1%) 9 1 (11.1%) 9 2 (6.3%) 32 Abdominal pain 1 (7.1%) 14 (%) 9 (%) 9 1 (3.1%) 32 Infection and infestations 2 (14.3%) 14 1 (11.1%) 9 (%) 9 3 (9.4%) 32 Nasopharyngitis 1 (7.1%) 14 1 (11.1%) 9 (%) 9 2 (6.3%) 32 Pneumonia 1 (7.1%) 14 (%) 9 (%) 9 1 (3.1%) 32 Herpes simplex 1 (7.1%) 14 (%) 9 (%) 9 1 (3.1%) 32 Hepatobiliary disorders (%) 14 (%) 9 2 (22.2%) 9 2 (6.3%) 32 Liver disorder (%) 14 (%) 9 1 (11.1%) 9 1 (3.1%) 32 Cholelithiasis (%) 14 (%) 9 1 (11.%) 9 1 (3.1%) 32 Eye disorders 1 (7.1%) 14 1 (11.1%) 9 (%) 9 2 (6.3%) 32 Eyelid oedema 1 (7.1%) 14 (%) 9 (%) 9 1 (3.1%) 32 Visual acuity reduced (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Musculoskeletal and connective tissue disorders (%) 14 (%) 9 3 (33.3%) 9 3 (9.4%) 32 Arthralgia (%) 14 (%) 9 2 (22.2%) 9 2 (6.3%) 32 Back pain (%) 14 (%) 9 1 (11.1%) 9 1 (3.1%) 32 Vascular disorders 1 (7.1%) 14 3 (33.3%) 9 1 (11.1%) 9 5 (15.6%) 32 Flushing (%) 14 3 (33.3%) 9 1 (11.1%) 9 4 (12.5%) 32 Hypertension 1 (7.1%) 14 1 (11.1%) 9 (%) 9 2 (6.3%) 32 Respiratory,thoracic and mediastinal disorders 1 (7.1%) 14 4 (44.4%) 9 1 (11.1%) 9 6 (18.8%) 32 Dyspnoea 1 (7.1%) 14 1 (11.1%) 9 1 (11.1%) 9 3 (9.4%) 32 Hypoxia (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Pharyngolaryngeal pain (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Upper respiratory tract inflammation (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Rhinalgia (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Cardiac disorders (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Tachycardia (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Nervous system disorders 2 (14.3%) 14 3 (33.3%) 9 4 (44.4%) 9 9 (28.1%) 32 Headache 1 (7.1%) 14 1 (11.1%) 9 2 (22.2%) 9 4 (12.5%) 32 Burning sensation 1 (7.1%) 14 (%) 9 1 (11.1%) 9 2 (6.3%) 32 Dizziness (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Cerebral infarction (%) 14 (%) 9 1 (11.1%) 9 1 (3.1%) 32 Nonketotic hyperglycaemic-hyperosmolar coma (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 General disorders and administration site conditions 4 (28.6%) 14 1 (11.1%) 9 4 (44.4%) 9 9 (28.1%) 32 Pyrexia 3 (21.4%) 14 1 (11.1%) 9 2 (22.2%) 9 6 (18.8%) 32 Chest pain 1 (7.1%) 14 1 (11.1%) 9 (%) 9 2 (6.3%) 32 Chills 1 (7.1%) 14 (%) 9 1 (11.1%) 9 2 (6.3%) 32 Asthenia (%) 14 (%) 9 1 (11.1%) 9 1 (3.1%) 32 Feeling cold (%) 14 (%) 9 1 (11.1%) 9 1 (3.1%) 32 Injection site anaesthesia 1 (7.1%) 14 (%) 9 (%) 9 1 (3.1%) 32 Metabolism and nutrition (%) 14 1 (11.1%) 9 2 (22.2%) 9 3 (9.4%) 32 disorders Anorexia (%) 14 (%) 9 2 (22.2%) 9 2 (6.3%) 32 Hyperglycaemia (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Endocrine disorders (%) 14 (%) 9 1 (11.1%) 9 1 (3.1%) 32 Hypothyroidism (%) 14 (%) 9 1 (11.1%) 9 1 (3.1%) 32 (%) 14 (%) 9 2 (22.2%) 9 2 (6.3%) 32 Pruritus (%) 14 (%) 9 2 (22.2%) 9 2 (6.3%) 32 Skin and subcutaneous tissue disorders Investigations 7 (5.%) 14 7 (77.8%) 9 9 (1%) 9 23 (71.9%) 32 White blood cell count decreased (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Neutrophil percentage decreased (%) 14 (%) 9 1 (11.1%) 9 1 (3.1%) 32 Eosinophil percentage decreased 1 (7.1%) 14 (%) 9 (%) 9 1 (3.1%) 32 Basophil percentage increased 1 (7.1%) 14 (%) 9 1 (11.1%) 9 2 (6.3%) 32 Basophil percentage decreased 1 (7.1%) 14 (%) 9 (%) 9 1 (3.1%) 32 Monocyte percentage increased 1 (7.1%) 14 (%) 9 1 (11.1%) 9 2 (6.3%) 32 Lymphocyte percentage decreased 1 (7.1%) 14 (%) 9 1 (11.1%) 9 2 (6.3%) 32 Differential white blood cell count abnormal (%) 14 (%) 9 1 (11.1%) 9 1 (3.1%) 32 Platelet count decreased (%) 14 1 (11.1%) 9 1 (11.1%) 9 2 (6.3%) 32 Blood sodium increased (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Blood potassium increased (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Blood potassium decreased 1 (7.1%) 14 3 (33.3%) 9 5 (55.6%) 9 9 (28.1%) 32 Blood magnesium increased (%) 12 (%) 9 1 (11.1%) 9 1 (3.3%) 3 Blood magnesium decreased (%) 12 2 (22.2%) 9 1 (11.1%) 9 3 (1.%) 3 Blood calcium increased (%) 14 (%) 9 1 (11.1%) 9 1 (3.1%) 32 Blood urea increased 1 (7.1%) 14 2 (22.2%) 9 4 (44.4%) 9 7 (21.9%) 32 Blood uric acid increased (%) 14 1 (11.1%) 9 (%) 9 1 (3.1%) 32 Blood uric acid decreased 1 (7.1%) 14 (%) 9 1 (11.1%) 9 2 (6.3%) 32 Blood creatinine increased 1 (7.1%) 14 4 (44.4%) 9 5 (55.6%) 9 1 (31.3%) 32 Blood bilirubin increased (%) 14 (%) 9 1 (11.1%) 9 1 (3.1%) 32 Aspatate aminotransferase increased (%) 14 1 (11.1%) 9 3 (33.3%) 9 4 (12.5%) 32 Alanine aminotransferase increased 1 (7.1%) 14 1 (11.1%) 9 2 (22.2%) 9 4 (12.5%) 32 Gamma-glutamyltransferase increased (%) 12 (%) 9 2 (22.2%) 9 2 (6.7%) 3
8 MedDRA SOC NAME Table4.Infusion-related reactions (IRRs) MedDRA PT NAME 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) Total number of patients 5 (35.7%) 3 (33.3%) 6 (66.7%) 14 (43.8%) Gastrointestinal disorders 1 (7.1%) 1 (11.1%) 3 (33.3%) 5 (15.6%) Nausea (%) 1 (11.1%) 3 (33.3%) 4 (12.5%) Vomiting (%) (%) 1 (11.1%) 1 (3.1%) Abdominal pain 1 (7.1%) (%) (%) 1 (3.1%) Eye disorders 1 (7.1%) (%) (%) 1 (3.1%) Eyelid oedema 1 (7.1%) (%) (%) 1 (3.1%) Musculoskeletal and connective tissue disorders (%) (%) 2 (22.2%) 2 (6.3%) Back pain (%) (%) 1 (11.1%) 1 (3.1%) Arthralgia (%) (%) 1 (11.1%) 1 (3.1%) Vascular disorders (%) 3 (33.3%) 1 (11.1%) 4 (12.5%) Flushing (%) 3 (33.3%) 1 (11.1%) 4 (12.5%) Hypertension (%) 1 (11.1%) (%) 1 (3.1%) Respiratory, thoracic and mediastinal disorders 1 (7.1%) 2 (22.2%) 1 (11.1%) 4 (12.5%) Dyspnoea 1 (7.1%) 1 (11.1%) 1 (11.1%) 3 (9.4%) Hypoxia (%) 1 (11.1%) (%) 1 (3.1%) Cardiac disorders (%) 1 (11.1%) (%) 1 (3.1%) Tachycardia (%) 1 (11.1%) (%) 1 (3.1%) Nervous system disorders 1 (7.1%) (%) 2 (22.2%) 3 (9.4%) Burning sensation 1 (7.1%) (%) 1 (11.1%) 2 (6.3%) Headache (%) (%) 1 (11.1%) 1 (3.1%) General disorders and administration site conditions 4 (28.6%) 1 (11.1%) (%) 5 (15.6%) Pyrexia 2 (14.3%) (%) (%) 2 (6.3%) Chest pain 1 (7.1%) 1 (11.1%) (%) 2 (6.3%) Chills 1 (7.1%) (%) (%) 1 (3.1%) Injection site anaesthesia 1 (7.1%) (%) (%) 1 (3.1%) meq/l 6. mg/dl Base line Mini mum End Follow -up Base line Maxi mum End Follow -up Fig.4a.Serum potassium level (median) in patients receiving L-AMB at doses of 1. mg/kg (), 2.5 mg/kg (), or 5. mg/ kg (). N13, 9 and 9 at doses of 1. mg/kg, 2.5 mg/kg, and 5. mg/kg. : P.5 (Signed rank test) Fig.4b.Serum creatinine level (median) in patients receiving L-AMB at doses of 1. mg/kg (), 2.5 mg/kg (), or 5. mg/ kg (). N13, 9 and 9 at doses of 1. mg/kg, 2.5 mg/kg, and 5. mg/kg. : P.5 (Signed rank test)
9 Table5a.AST (GOT) levels in patients receiving L-AMB at a dose of 1. mg/kg (n13), 2.5 mg/kg (n9), or 5. mg/kg (n9) Baseline Maximum End Follow-up Median Min Max Median Min Max Median Min Max Median Min Max 1. mg/kg mg/kg mg/kg Table5b.ALT (GPT) levels in patients receiving L-AMB at a dose of 1. mg/kg (n13), 2.5 mg/kg (n9), or 5. mg/kg (n9) Baseline Maximum End Follow-up Median Min Max Median Min Max Median Min Max Median Min Max 1. mg/kg mg/kg mg/kg Macaca mulatta
10
11 μ μ
日本化学療法学会雑誌第60巻第4号
 Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Key words β Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae μ μ S. pneumoniae H. influenzae S. pneumoniae
Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Key words β Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae μ μ S. pneumoniae H. influenzae S. pneumoniae
日本化学療法学会雑誌第65巻第3号
 μ Key words Chlamydia trachomatis C. trachomatis I Table1.Observation items, categories and scores Observation item Category Score Body temperature () Lower abdominal pain Uterine corpus tenderness Condition
μ Key words Chlamydia trachomatis C. trachomatis I Table1.Observation items, categories and scores Observation item Category Score Body temperature () Lower abdominal pain Uterine corpus tenderness Condition
日本化学療法学会雑誌第51巻第2号
 piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
日本化学療法学会雑誌第59巻第5号
 Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
日本化学療法学会雑誌第59巻第6号
 Key words Survey forms collected: 32,200 Patients subject to safety analysis: 29,880 Excluded from safety analysis: 2,320 Reasons: Administration outside contract period: 6 Registered 8 days after the
Key words Survey forms collected: 32,200 Patients subject to safety analysis: 29,880 Excluded from safety analysis: 2,320 Reasons: Administration outside contract period: 6 Registered 8 days after the
日本化学療法学会雑誌第58巻第2号
 Key words β Candida Table1. MCFGCPAstudygroup Investigator (representative) YoshitsuguMiyazaki NaoyukiMiyashita RyoichiAmitani KenjiOgawa AtsuyukiKurashima ToshiroKiguchi MichiakiMishima YuichiInoue HiroshiSaito
Key words β Candida Table1. MCFGCPAstudygroup Investigator (representative) YoshitsuguMiyazaki NaoyukiMiyashita RyoichiAmitani KenjiOgawa AtsuyukiKurashima ToshiroKiguchi MichiakiMishima YuichiInoue HiroshiSaito
日本化学療法学会雑誌第54巻第S-1号
 β β β Key words β β I HP- -CD CHR RHC R R R R CHR R R R R RHC R R R CHR R R R RHC CHR R H CH CH Fig.. Structuralformulaofhydroxypropyl-β -cyclodextrin(hp-β -CD). CH n H β β β β Table-. Dosageandadministration(Singleadministrationstudy)
β β β Key words β β I HP- -CD CHR RHC R R R R CHR R R R R RHC R R R CHR R R R RHC CHR R H CH CH Fig.. Structuralformulaofhydroxypropyl-β -cyclodextrin(hp-β -CD). CH n H β β β β Table-. Dosageandadministration(Singleadministrationstudy)
DatRetPrePTC_R1.4_J.doc
 MedDRA Release 1.4 MedDRA 10.1 ICH MedDRA 2007 912 MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER Release 1.4 Based on MedDRA Version 10.1 ICH-Endorsed Guide for MedDRA Users on Data Output
MedDRA Release 1.4 MedDRA 10.1 ICH MedDRA 2007 912 MedDRA DATA RETRIEVAL AND PRESENTATION: POINTS TO CONSIDER Release 1.4 Based on MedDRA Version 10.1 ICH-Endorsed Guide for MedDRA Users on Data Output
CHEMOTHERAPY APR. 1982
 VOL.30 S-1 CHEMOTHERAPY Table 1 Dose of CTT and subjects i. v.: Intravenous bolus injection d. i. v.: Intravenous drip infusion i. m.: Intramuscular injection Fig. 1 Schedule for examination of CTT, 1.0g
VOL.30 S-1 CHEMOTHERAPY Table 1 Dose of CTT and subjects i. v.: Intravenous bolus injection d. i. v.: Intravenous drip infusion i. m.: Intramuscular injection Fig. 1 Schedule for examination of CTT, 1.0g
日本化学療法学会雑誌第56巻第1号
 β β β β β Streptococcus pneumoniaehaemophilus influenzaemoraxella catarrhalismycoplasma pneumoniaechlamydia pneumoniae β Key wordsβ mys nilc laci ngis Table. Assessmentschedule Parameters Patientcharacteristics
β β β β β Streptococcus pneumoniaehaemophilus influenzaemoraxella catarrhalismycoplasma pneumoniaechlamydia pneumoniae β Key wordsβ mys nilc laci ngis Table. Assessmentschedule Parameters Patientcharacteristics
日本化学療法学会雑誌第57巻第S-2号
 Key words β I Table. Observationandtestschedule Testschedule Observation/Tests Beforetreatment Endoftreatment 5 9days aftercompletion oftreatment 4 6weeks aftercompletion oftreatment Informedconsent Patientbackground
Key words β I Table. Observationandtestschedule Testschedule Observation/Tests Beforetreatment Endoftreatment 5 9days aftercompletion oftreatment 4 6weeks aftercompletion oftreatment Informedconsent Patientbackground
CHEMOTHERAPY JUN Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter cloacae 27, Proteus rettgeri 7, Proteus inconstans 20, Proteus
 VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
VOL.39 S-3
 VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
988 CHEMOTHERAPY NOV. 1971
 988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
CHEMOTHERAPY Fig. 1 Chemical structure of CXM-AX
 Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
日本化学療法学会雑誌第56巻第3号
 β Key words Candida albicans albicans Candida C. albicans Candida glabrata Candida krusei albicans Candida C. glabrata C. albicans albicans Candida β in vitro in vivo C. glabrata C. krusei I β γ µ Candida
β Key words Candida albicans albicans Candida C. albicans Candida glabrata Candida krusei albicans Candida C. glabrata C. albicans albicans Candida β in vitro in vivo C. glabrata C. krusei I β γ µ Candida
日本化学療法学会雑誌第57巻第S-2号
 µ µ Key words µ µ µ I Table1. Subjectprofilesin(A)singleand(B)multiple-dosestudies (A)Single-dosestudy Age (yr) Height (cm) Bodyweight (kg) BMI (kg/m ) Ccr(Cockcroft) (ml/min) Ethnicity dose(mg) n 5 5.1±.,3
µ µ Key words µ µ µ I Table1. Subjectprofilesin(A)singleand(B)multiple-dosestudies (A)Single-dosestudy Age (yr) Height (cm) Bodyweight (kg) BMI (kg/m ) Ccr(Cockcroft) (ml/min) Ethnicity dose(mg) n 5 5.1±.,3
L-DOPA III ( ( [ ] UPDRS Part III Observed Case(OC UPDRS Part III 4.05 OC Last Observation Carried Forward(LOCF S.D
![L-DOPA III ( ( [ ] UPDRS Part III Observed Case(OC UPDRS Part III 4.05 OC Last Observation Carried Forward(LOCF S.D L-DOPA III ( ( [ ] UPDRS Part III Observed Case(OC UPDRS Part III 4.05 OC Last Observation Carried Forward(LOCF S.D](/thumbs/89/98293599.jpg) 248.505 (3 III L-DOPA 6, 7 1 III L-DOPA ( 248.505 6 L-DOPA L-DOPA III 248.326 7 87 87L-DOPA III ( 248.505 L-DOPA 3 L-DOPA (1 120 2 3 Modified Hoehn & Yahr (2L-DOPA 1Wearing-off 2on-off 3 L-DOPA 4L-DOPA
248.505 (3 III L-DOPA 6, 7 1 III L-DOPA ( 248.505 6 L-DOPA L-DOPA III 248.326 7 87 87L-DOPA III ( 248.505 L-DOPA 3 L-DOPA (1 120 2 3 Modified Hoehn & Yahr (2L-DOPA 1Wearing-off 2on-off 3 L-DOPA 4L-DOPA
 VOL.32 S-9 CHEMOTHERAPY Table 1 Minimum inhibitory concentrations of AC-1370, CPZ and CAZ Table 2 Efficacy of AC-1370 and CPZ against systemic infections in mice *Inoculum size: 106 cells/ml * 95% confidence
VOL.32 S-9 CHEMOTHERAPY Table 1 Minimum inhibitory concentrations of AC-1370, CPZ and CAZ Table 2 Efficacy of AC-1370 and CPZ against systemic infections in mice *Inoculum size: 106 cells/ml * 95% confidence
 2) Dolphin, A., Jenner, P., Marsden, C,D., Pycock, C. and Tarsy, D. : Pharmacological evidence for cerebral dopamine receptor blockade by metoclopramide in rodents, Psychopharmacologia, 41 : 133, 1975.
2) Dolphin, A., Jenner, P., Marsden, C,D., Pycock, C. and Tarsy, D. : Pharmacological evidence for cerebral dopamine receptor blockade by metoclopramide in rodents, Psychopharmacologia, 41 : 133, 1975.
Jan THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Table 1. Outline of administering doses, routes and sampling times *: 4 ml/hr/kg Bacillus subtilis
 THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Jan. 1987 Jan. 1987 THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Table 1. Outline of administering doses, routes and sampling times *: 4 ml/hr/kg Bacillus subtilis
THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Jan. 1987 Jan. 1987 THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Table 1. Outline of administering doses, routes and sampling times *: 4 ml/hr/kg Bacillus subtilis
 600mg 600mg CTD 2 2.5 2.5 Page 3 2.5...7 2.5.1...7 2.5.2...27 2.5.3...28 2.5.4...42 2.5.5...55 2.5.6...79 2.5.7...97 2.5 Page 5 73 67 31 48 48A 102 104 105 106 ALP ALT(GPT) AST(GOT) AUC AUEC BID BUN
600mg 600mg CTD 2 2.5 2.5 Page 3 2.5...7 2.5.1...7 2.5.2...27 2.5.3...28 2.5.4...42 2.5.5...55 2.5.6...79 2.5.7...97 2.5 Page 5 73 67 31 48 48A 102 104 105 106 ALP ALT(GPT) AST(GOT) AUC AUEC BID BUN
CHEMOTHERAPY FEB Table 1 Background of volunteers
 CHEMOTHERAPY FEB. 1985 Table 1 Background of volunteers Table 3 Reproducibility of saisomicln In the EIA and the RIA Fig.1 Comparison of the EIA with the RIA or bioassay of sisomicin Table 4 Blood levels
CHEMOTHERAPY FEB. 1985 Table 1 Background of volunteers Table 3 Reproducibility of saisomicln In the EIA and the RIA Fig.1 Comparison of the EIA with the RIA or bioassay of sisomicin Table 4 Blood levels
Fig. 1 Chemical structure of norfioxacin (AM-715)
 Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
125 2 P 1st washout 2 PB P mg/dL nd washout 2 P 5.5mg/dL< mg/dL <2.5mg/dL P P 2 D D 3 Ca 10
 . (1) 125 1 125 Renagel PB-94 P intact-pth P 1 b c a b 1 18 2 3 3 3 1 P 4 D 1 5 6 7 2 1HIV 2 3 4 5Hb 8.0g/dL ALT 48IU/L 6 7 PB-94 440mg 403mg 1-196 125 2 P 1st washout 2 PB-94 1 2 4 4 P 2.5 5.5mg/dL 1
. (1) 125 1 125 Renagel PB-94 P intact-pth P 1 b c a b 1 18 2 3 3 3 1 P 4 D 1 5 6 7 2 1HIV 2 3 4 5Hb 8.0g/dL ALT 48IU/L 6 7 PB-94 440mg 403mg 1-196 125 2 P 1st washout 2 PB-94 1 2 4 4 P 2.5 5.5mg/dL 1
 Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
400 46 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 Dec. 2012 LVFX 100 mg 3 / 7 150 mg 2 / 7 2 2006 2008 9 LVFX PK PD 2009 7 100 mg 1 3 500 mg 1 1 AUC/MIC
 Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels of mezlocillin
 CHEMOTHERAPY Fig. 2 Urinary excretion of mezlocillin Fig. 1 Blood levels of mezlocillin CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels
CHEMOTHERAPY Fig. 2 Urinary excretion of mezlocillin Fig. 1 Blood levels of mezlocillin CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S.
 Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
Vol. 36, Special Issue, S 3 S 18 (2015) PK Phase I Introduction to Pharmacokinetic Analysis Focus on Phase I Study 1 2 Kazuro Ikawa 1 and Jun Tanaka 2
 Vol. 36, Special Issue, S 3 S 18 (2015) PK Phase I Introduction to Pharmacokinetic Analysis Focus on Phase I Study 1 2 Kazuro Ikawa 1 and Jun Tanaka 2 1 2 1 Department of Clinical Pharmacotherapy, Hiroshima
Vol. 36, Special Issue, S 3 S 18 (2015) PK Phase I Introduction to Pharmacokinetic Analysis Focus on Phase I Study 1 2 Kazuro Ikawa 1 and Jun Tanaka 2 1 2 1 Department of Clinical Pharmacotherapy, Hiroshima
Fig. 1 Chemical structure of KW-1070
 Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
CHEMOTHERAPY JUNE 1988 ( })-1-ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-l-piperaziny1) 4-oxo-3-quinolinecarboxylic acid hydrochloride Fig. 1. Chemica
 CHEMOTHERAPY JUNE 1988 ( })-1-ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-l-piperaziny1) 4-oxo-3-quinolinecarboxylic acid hydrochloride Fig. 1. Chemical structure of NY-198 VOL. 36 5-2 CHEMOTHERAPY Mouse
CHEMOTHERAPY JUNE 1988 ( })-1-ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-l-piperaziny1) 4-oxo-3-quinolinecarboxylic acid hydrochloride Fig. 1. Chemical structure of NY-198 VOL. 36 5-2 CHEMOTHERAPY Mouse
CHEMOTHERAPY
 CHEMOTHERAPY VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin CHEMOTHERAPY AUG. 1993 VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin Table 1. Comparative in vitro activity of teicoplanin
CHEMOTHERAPY VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin CHEMOTHERAPY AUG. 1993 VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin Table 1. Comparative in vitro activity of teicoplanin
 Ⅰ. Statistics Concerning Health Birthweight (1) Birthweight by year (2) Mean birthweight by prefecture, 2007-1997 (3) Percentage of births under 2,500g by prefecture, 2007-1997 Sex Ratio (4) Sex ratio
Ⅰ. Statistics Concerning Health Birthweight (1) Birthweight by year (2) Mean birthweight by prefecture, 2007-1997 (3) Percentage of births under 2,500g by prefecture, 2007-1997 Sex Ratio (4) Sex ratio
VOL. 36 S-3 CHEMOTHERAPY 437
 VOL. 36 S-3 CHEMOTHERAPY 437 438 CHEMOTHERAPY JULY 1988 Fig. 1 Contractile response of gastrointestinal tract to intravenous administration of saline and EM in interdigestive state in dogs (a) : Saline,
VOL. 36 S-3 CHEMOTHERAPY 437 438 CHEMOTHERAPY JULY 1988 Fig. 1 Contractile response of gastrointestinal tract to intravenous administration of saline and EM in interdigestive state in dogs (a) : Saline,
 9) Reichen J, Paumgartner G: Relationship be- tween bile flow and Na+, K+-Adenosinetriphos- phatase in liver plasma membranes enriched in bile canaliculi. J Clin Invest 60: 429-434, 1977 10) Schaffner
9) Reichen J, Paumgartner G: Relationship be- tween bile flow and Na+, K+-Adenosinetriphos- phatase in liver plasma membranes enriched in bile canaliculi. J Clin Invest 60: 429-434, 1977 10) Schaffner
ダラザレックス点滴静注 100 mg ダラザレックス点滴静注 400 mg に関する資料 本資料に記載された情報に係る権利及び内容についての責任は ヤンセンファーマ株式会社に帰属するものであり 当該情報を本薬剤の適正使用以外の営利目的に使用することはできません ヤンセンファーマ株式会社
 ダラザレックス点滴静注 100 mg ダラザレックス点滴静注 400 mg に関する資料 本資料に記載された情報に係る権利及び内容についての責任は ヤンセンファーマ株式会社に帰属するものであり 当該情報を本薬剤の適正使用以外の営利目的に使用することはできません ヤンセンファーマ株式会社 1.5 1.5... 3 1 1.5 DVd MM Rd Vd Daratumumab-bortezomib-dexamethasone
ダラザレックス点滴静注 100 mg ダラザレックス点滴静注 400 mg に関する資料 本資料に記載された情報に係る権利及び内容についての責任は ヤンセンファーマ株式会社に帰属するものであり 当該情報を本薬剤の適正使用以外の営利目的に使用することはできません ヤンセンファーマ株式会社 1.5 1.5... 3 1 1.5 DVd MM Rd Vd Daratumumab-bortezomib-dexamethasone
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, Sc
 A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
untitled
 1988 2000 2002 2004 2006 2008 IFN Lamivudine Adefovir Entecavir 1 Total number 560 Sex (male/female) 424/136 Age (years)* 38 (15-68) Duration of treatment (weeks)* 26 (1-592) Follow-up time (years) 75(05-21
1988 2000 2002 2004 2006 2008 IFN Lamivudine Adefovir Entecavir 1 Total number 560 Sex (male/female) 424/136 Age (years)* 38 (15-68) Duration of treatment (weeks)* 26 (1-592) Follow-up time (years) 75(05-21
Fig. 1 Chemical structure of DL-8280
 Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
2108 CHEMOTHERAPY SEPT Table 1 Antimicrobial spectrum Fig. 1
 2108 CHEMOTHERAPY SEPT. 1977 Table 1 Antimicrobial spectrum Fig. 1 VOL. 25 NO. 7 CHEM 014 HERAPY 2109 Table 2 Susceptibility distribution of Staphylococcus aureus to aminoglycosides (54 strains) Table
2108 CHEMOTHERAPY SEPT. 1977 Table 1 Antimicrobial spectrum Fig. 1 VOL. 25 NO. 7 CHEM 014 HERAPY 2109 Table 2 Susceptibility distribution of Staphylococcus aureus to aminoglycosides (54 strains) Table
 M1 TSH T 3 T 4 2 T 1 2 Plummer 3 TSH TSH K 5 7 TSH TSH Plummer TSH TSH TSH TSH T 4 T 3 TSH T 4 T 3 TSH TSH or Plummer Plummer or a) 1. 2. 3. b) 1. T 4 T 3 2. TSH 0.1 U/ml 3. TSH TRAb TSAb 4. 1) a) 1
M1 TSH T 3 T 4 2 T 1 2 Plummer 3 TSH TSH K 5 7 TSH TSH Plummer TSH TSH TSH TSH T 4 T 3 TSH T 4 T 3 TSH TSH or Plummer Plummer or a) 1. 2. 3. b) 1. T 4 T 3 2. TSH 0.1 U/ml 3. TSH TRAb TSAb 4. 1) a) 1
CHEMOTHERAPY FEB Table 1. Activity of cefpirome and others against clinical isolates
 VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
VOL.35 S-2 CHEMOTHERAPY Table 1 Sex and age distribution Table 2 Applications of treatment with carumonam Table 3 Concentration of carumonam in human
 CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution of female patients with blad
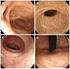 Key words: Female with bladder irritability, Subjective symptoms, Pyuria, Bacteriuria Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution
Key words: Female with bladder irritability, Subjective symptoms, Pyuria, Bacteriuria Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution
広報さがみはら第1242号
 LINE UP 3 1 5 6 1 NO.1242 S A G A M I H A R A 1 1 1 16 16 1 6 1 6 1 6 1 1 1 1 1 11 1 1 1 1 1 1 6 1 6 1 1 1 1 1 1 1 1 11 1 1 16 1 1 1 6 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 6 1 16 1 16 1 6 1 1 1 1 1 1
LINE UP 3 1 5 6 1 NO.1242 S A G A M I H A R A 1 1 1 16 16 1 6 1 6 1 6 1 1 1 1 1 11 1 1 1 1 1 1 6 1 6 1 1 1 1 1 1 1 1 11 1 1 16 1 1 1 6 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 1 6 1 16 1 16 1 6 1 1 1 1 1 1
 Clinical Characteristics in Multiple Endocrine Neoplasia Type 1 in Japan: A review of 106 patients Katsuhiko YOSHIMOTO 1,2, Shiro SAITO 1 1. The First Department of Internal Medicine 2. Otsuka Department
Clinical Characteristics in Multiple Endocrine Neoplasia Type 1 in Japan: A review of 106 patients Katsuhiko YOSHIMOTO 1,2, Shiro SAITO 1 1. The First Department of Internal Medicine 2. Otsuka Department
 Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST
 Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
日本化学療法学会雑誌第57巻第4号
 Streptococcus pneumoniae Haemophilus influenzae β β Key words I β Enterococcus faecium Pseudomonas aeruginosa Streptococcus pneumoniae S. pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Table.
Streptococcus pneumoniae Haemophilus influenzae β β Key words I β Enterococcus faecium Pseudomonas aeruginosa Streptococcus pneumoniae S. pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Table.
1996 2000 2004 1984 2005 7150 000 9 500 9 4 13 10 95 11 11 12 20002004 9 70
 14 2006 1 Key Words 2002 3 1 2 3 3 1 2 3 1969 1987 69 1996 2000 2004 1984 2005 7150 000 9 500 9 4 13 10 95 11 11 12 20002004 9 70 14 2006 1 15 71 72 1 22 6 32 9 200 6 3 1 2 2000 10 1 2003 10 2005 6 5 4
14 2006 1 Key Words 2002 3 1 2 3 3 1 2 3 1969 1987 69 1996 2000 2004 1984 2005 7150 000 9 500 9 4 13 10 95 11 11 12 20002004 9 70 14 2006 1 15 71 72 1 22 6 32 9 200 6 3 1 2 2000 10 1 2003 10 2005 6 5 4
立命館21_川端先生.indd
 21 119-132 2010 ( ) ' Key Words 119 21 2010 7 1962 2001 2001 2007 1982 1988 1997 2007 1997 1998 1863 1880 1 1998 1998 2001 1599 120 121 1599 1695 8 1695 1714 4 1714 1715 5 1715 100 1812 9 1812 1864 2001
21 119-132 2010 ( ) ' Key Words 119 21 2010 7 1962 2001 2001 2007 1982 1988 1997 2007 1997 1998 1863 1880 1 1998 1998 2001 1599 120 121 1599 1695 8 1695 1714 4 1714 1715 5 1715 100 1812 9 1812 1864 2001


