日本化学療法学会雑誌第65巻第3号
|
|
|
- やすもり かなり
- 5 years ago
- Views:
Transcription
1 μ Key words
2 Chlamydia trachomatis C. trachomatis I
3 Table1.Observation items, categories and scores Observation item Category Score Body temperature () Lower abdominal pain Uterine corpus tenderness Condition of vaginal discharge Amount of vaginal discharge WBC (/mm 3 ) CRP (mg/dl) Intensive (3) 3 Moderate (2) 2 Mild (1) 1 None 0 Intensive (3) 3 Moderate (2) 2 Mild (1) 1 None 0 Purulent 4 Serosity 2 Normal or None 0 2 ml 3 12 ml 2 1 ml 1 None 0 12, ,00012, ,00010, , β β Escherichia coliklebsiella pneumoniaeklebsiella oxytoca Proteus mirabilis Enterobacter Citrobacter Serratia Pseudomonas aeruginosa C. trachomatis Neisseria gonorrhoeae γ
4
5 Table2.Subject characteristics (1) Characteristics Intrauterine infection Uterine adnexitis Total Number of subjects evaluated n7 n12 n19 Age (yr) (28.6) 8 (66.7) 10 (52.6) (57.1) 4 (33.3) 8 (42.1) 60 1 (14.3) 0 (0.0) 1 (5.3) MeanSD Median [Min. Max.] [3164] [2146] [2164] Body weight (kg) 40 0 (0.0) 0 (0.0) 0 (0.0) (85.7) 8 (66.7) 14 (73.7) (14.3) 4 (33.3) 5 (26.3) 80 0 (0.0) 0 (0.0) 0 (0.0) MeanSD Median [Min. Max.] [ ] [ ] [ ] Ccr (ml/min) 50 0 (0.0) 0 (0.0) 0 (0.0) (0.0) 1 (8.3) 1 (5.3) 80 7 (100.0) 11 (91.7) 18 (94.7) MeanSD Median [Min. Max.] [ ] [ ] [ ] Previous antimicrobial treatment No 4 (57.1) 9 (75.0) 13 (68.4) Yes 3 (42.9) 3 (25.0) 6 (31.6) Causative organism No 0 (0.0) 1 (8.3) 1 (5.3) Yes 7 (100.0) 11 (91.7) 18 (94.7) ( ): %, Ccr: Creatinine clearance (Ccr was estimated using the Cockcroft-Gault formula.) II E. coli Haemophilus influenzae Peptoniphilus asaccharolyticus Peptostreptococcus anaerobius Finegoldia magna Enterococcus faecalis Streptococcus agalactiae P. asaccharolyticus F. magna P. anaerobius Prevotella bivia E. coli
6 Table3.Subject characteristics (2) Characteristics Intrauterine infection Uterine adnexitis Total Number of subjects evaluated n7 n12 n19 Body temperature () (0.0) 1 (8.3) 1 (5.3) (57.1) 7 (58.3) 11 (57.9) (28.6) 2 (16.7) 4 (21.1) (14.3) 2 (16.7) 3 (15.8) MeanSD Median [Min. Max.] [ ] [ ] [ ] WBC (/mm 3 ) 8,000 0 (0.0) 4 (33.3) 4 (21.1) 8,00010,000 0 (0.0) 2 (16.7) 2 (10.5) 10,00012,000 2 (28.6) 1 (8.3) 3 (15.8) 12,000 5 (71.4) 5 (41.7) 10 (52.6) MeanSD 14,377.13, ,939.23, ,205.84,074.4 Median 14, , ,000.0 [Min. Max.] [10,60020,300] [6,50019,000] [6,50020,300] CRP (mg/ml) (14.3) 1 (8.3) 2 (10.5) (42.9) 5 (41.7) 8 (42.1) (14.3) 3 (25.0) 4 (21.1) (28.6) 3 (25.0) 5 (26.3) MeanSD Median [Min. Max.] [ ] [ ] [ ] Lower abdominal pain Intensive (3) 0 (0.0) 0 (0.0) 0 (0.0) Moderate (2) 5 (71.4) 11 (91.7) 16 (84.2) Mild (1) 2 (28.6) 0 (0.0) 2 (10.5) None 0 (0.0) 1 (8.3) 1 (5.3) Uterine corpus tenderness Condition of vaginal discharge Amount of vaginal discharge ( ): % Intensive (3) 0 (0.0) 0 (0.0) 0 (0.0) Moderate (2) 5 (71.4) 11 (91.7) 16 (84.2) Mild (1) 2 (28.6) 0 (0.0) 2 (10.5) None 0 (0.0) 1 (8.3) 1 (5.3) Purulent 5 (71.4) 3 (25.0) 8 (42.1) Serosity 2 (28.6) 8 (66.7) 10 (52.6) Normal or None 0 (0.0) 0 (0.0) 0 (0.0) 2 ml 3 (42.9) 4 (33.3) 7 (36.8) 12 ml 3 (42.9) 3 (25.0) 6 (31.6) 1 ml 1 (14.3) 4 (33.3) 5 (26.3) None 0 (0.0) 0 (0.0) 0 (0.0)
7 Table4.Baseline causative organisms identified Causative organisms Intrauterine infection Uterine adnexitis Total Number of subjects evaluated n7 n12 n19 Monomicrobial infection 1 (14.3) 5 (41.7) 6 (31.6) Gram-positive bacteria Staphylococcus heamolyticus 1 1 Streptococcus agalactiae 1 1 Anaerobic bacteria Finegoldia magna 1 1 Peptostreptococcus prevotii 1 1 Gram-positive rod anaerobic 1 1 Atypical bacteria Chlamydia trachomatis 1 1 Polymicrobial infection 6 (85.7) 6 (50.0) 12 (63.2) Two organisms Three organisms Four organisms Five organisms 1 1 Six organisms Pathogens were unknown 0 (0.0) 1 (8.3) 1 (5.3) ( ): % Table5.Distribution of causative organisms Causative organisms Intrauterine infection Uterine adnexitis Total Number of subjects evaluated n7 n11 n18 Gram-positive bacteria Staphylococcus aureus (MSSA) 1 1 Staphylococcus haemolyticus 1 1 Streptococcus lugdunensis 1 1 Streptococcus agalactiae hemolytic Streptococcus 1 1 Enterococcus faecalis Enterococcus avium 1 1 Enterococcus raffinosus 1 1 Gram-negative bacteria Neisseria gonorrhoeae 1 1 Escherichia coli Heamophilus influenzae Anaerobic bacteria Peptoniphilus asaccharolyticus Peptostreptococcus anaerobius Finegoldia magna Peptostreptococcus prevotii 1 1 Bacteroides thetaiotaomicron Bacteroides vulgatus 1 1 Prevotella sp. 1 1 Prevotella bivia 2 2 Porphyromonas asaccharolytica 1 1 Fusobacterium sp. 1 1 Gram-positive rod anaeribic Atypical bacteria Chlamydia trachomatis Total
8 Visit Diagnosis n EOT Table6.Clinical response Clinical response Efficacy rate (%) (95% CI) Cured Improved Failed Unknown Intrauterine infection (59.8, 100.0) Uterine adnexitis (76.0, 100.0) All patients (75.7, 100.0) Intrauterine infection (59.8, 100.0) TOC Uterine adnexitis (55.2, 100.0) All patients (64.2, 100.0) Efficacy rate(curedimproved)/(nunknown)100 EOT: End of treatment; TOC: Test of cure Table7.Clinical response of LVFX intravenous-to-oral sequential therapy Diagnosis Route of administration n Clinical response (TOC) Cured Improved Failed Unknown Efficacy rate (%) Intrauterine infection LVFX IV-to-oral sequential therapy LVFX IV only /2 Uterine adnexitis LVFX IV-to-oral sequential therapy LVFX IV only /2 Efficacy rate(curedimproved)/(nunknown)100 TOC: Test of cure Table8.Clinical response assessed by the principal investigator Diagnosis n Clinical response (EOT) Efficacy rate (%) Cured Failed Unknown (95% CI) Intrauterine infection (59.8, 100.0) Uterine adnexitis (76.0, 100.0) All patients (75.7, 100.0) Efficacy ratecured/ (nunknown)100 EOT: End of treatment Total score (symptoms and laboratory findings) (A) (B) Outlier Max 75% file Average Median 25% file Min Day 0 EOT TOC Day 0 EOT TOC vs. Day 0 (Wilcoxon signed-rank test) p p p p Fig.1.Changes in the total score. (A) intrauterine infection (n7), (B) uterine adnexitis (n12) EOT: End of treatment, TOC: Test of cure
9 Total score (symptoms and laboratory findings) (A) (B) Outlier Max 75% file Average Median 25% file Min Day 0 EOI EOT TOC Day 0 EOI EOT TOC vs. Day 0 (Wilcoxon signed-rank test) p p p p p p Fig.2.Changes in the total score (The patients who did the LVFX intravenous-to-oral switching therapy). (A) intrauterine infection (n5), (B) uterine adnexitis (n9) EOI: End of intravenous treatment, EOT: End of treatment, TOC: Test of cure Diagnosis n Eradication Table9.Microbiological response at end of treatment Microbial substitution Microbiological response Replacement bacterium Persistance Unknown Eradication rate (%) (95% CI) Intrauterine infection (6.2, 79.5) Uterine adnexitis (59.0, 100.0) All patients (44.9, 88.4) Eradication rate (%) (EradicationMicrobial substitution)/(nunknown)100 C. trachomatis E. faecalis μ N. gonorrhoeae μ P. asaccharolyticus μ μ P. anaerobius μ μ F. magna μ Bacteroides thetaiotaomicron μ Porphyromonas asaccharolytica μ μ
10 Table10.Microbiological response by MIC at end of treatment LVFX MIC (g/ml) Causative organisms N. D. N. C. Total Gram-positive bacteria Staphylococcus aureus (MSSA) 1/1 1/1 Staphylococcus haemolyticus 1/1 1/1 Staphylococcus lugdunensis 1/1 1/1 Streptococcus agalactiae 1/1 2/2 3/3 -hemolytic Streptococcus 1/1 1/1 Enterococcus faecalis 1/2 2/2 3/4 Enterococcus avium 1/1 1/1 Enterococcus raffinosus 1/1 1/1 Gram-negative bacteria Neisseria gonorrhoeae 0/1 0/1 Escherichia coli 2/2 1/1 3/3 Haemophilus influenzae 3/3 3/3 Anaerobic bacteria Peptoniphilus asaccharolyticus 1/1 1/3 1/2 3/6 Peptostreptococcus anaerobius 1/1 0/1 1/2 2/4 Finegoldia magna 1/1 3/3 0/1 4/5 Peptostreptococcus prevotii 1/1 1/1 Bacteroides thetaiotaomicron 0/1 1/1 1/2 Bacteroides vulgatus 1/1 1/1 Prevotella sp. 1/1 1/1 Prevotella bivia 1/1 1/1 2/2 Porphyromonas asaccharolytica 0/1 0/1 Fusobacterium sp. 1/1 1/1 Gram-positive rod anaerobic 2/2 2/2 Atypical bacteria Chlamydia trachomatis 1/2 1/2 Total 5/5 2/2 1/1 3/4 5/6 4/4 5/5 2/5 1/2 0/1 5/6 2/4 1/1 1/2 37/48 N.D.: Not done, N.C.: Not calculated LVFX concentration (g/ml or g/g) Actual time (h) Fig.3.Concentration of LVFX in plasma and vaginal discharge (LVFX injection 500 mg/60 min infusion). : plasma, : vaginal discharge μ III
11 End of infusion Sampling 37 h after starting infusion 1724 h after starting infusion Table11.Concentration of LVFX in plasma and vaginal discharge plasma (g/ml) LVFX concentration vaginal discharge (g/g) Penetration ratio n 8 MeanSD Median 10.5 Min, Max 9.11, n MeanSD Median Min, Max 5.40, , , 2.21 n 8 MeanSD Median 0.74 Min, Max 0.30, 1.71 Penetration ratiovaginal discharge concentration/plasma concentration N. gonorrhoeaec. trachomatis N. gonorrhoeaec. trachomatis N. gonorrhoeae N. gonorrhoeae μ E. faecalis μ P. asaccharolytica μ P. asacchalyticus μ B. thetaiotaomicron μ Peptostreptococcus prevottii μ C. trachomatis C. trachomatis C. trachomatis C. trachomatis C. trachomatis S. agalactiaee. faecalisenterococcus aviume. coli P. asaccharolyticusp. anaerobius F. magna
12 Table12.Adverse event and adverse drug reaction incidence Adverse event Adverse drug reaction Patients evaluated for safety Patients with adverse event/drug reaction (%) 16 (76.2) 8 (38.1) Events System organ class and Prefered term Patients (%) Events Patients (%) Events Infections and infestations Genital herpes 1 (4.8) 1 Nasopharyngitis 1 (4.8) 1 Vulvovaginal candidiasis 1 (4.8) 1 1 (4.8) 1 Blood and lymphatic system disorders Anaemia 1 (4.8) 1 Iron deficiency anaemia 1 (4.8) 1 Metabolism and nutrition disorders Decreased appetite 1 (4.8) 1 Psychiatric disorders Insomnia 3 (14.3) 6 Nervous system disorders Dizziness 1 (4.8) 1 Dysgeusia 1 (4.8) 1 Headaches 1 (4.8) 1 Vascular disorders Hot flush 1 (4.8) 1 Gastrointestinal disorders Diarrhoea 3 (14.3) 3 2 (9.5) 2 Constipation 2 (9.5) 2 Abdominal discomfort 1 (4.8) 1 1 (4.8) 1 Abdominal pain 1 (4.8) 1 Abdominal pain lower 1 (4.8) 1 Apical cyst 1 (4.8) 1 Vomiting 1 (4.8) 1 Skin and subcutaneous tissue disorders Rash 2 (9.5) 2 Erythema 1 (4.8) 1 Pruritus 1 (4.8) 1 Musculoskeletal and connective tissue disorders Back pain 1 (4.8) 1 Renal and urinary disorders Pollakiuria 1 (4.8) 1 Urethral pain 1 (4.8) 1 General disorders and administration site conditions Injection site pain 2 (9.5) 6 1 (4.8) 5 Fatigue 1 (4.8) 1 Investigations Alanine aminotransferase increased 3 (14.3) 3 3 (14.3) 3 Aspartate aminotransferase increased 3 (14.3) 3 3 (14.3) 3 Blood billirubin increased 1 (4.8) 1 1 (4.8) 1 Blood lactate dehydrogenase increased 1 (4.8) 1 1 (4.8) 1 Gamma-glutamyltransferase increased 1 (4.8) 1 1 (4.8) 1 White blood cell count increased 1 (4.8) 1 1 (4.8) 1 Blood alkaline phosphatase increased 1 (4.8) 1 1 (4.8) 1 MedDRA/J version 16.1 μ μ E. faecalisn. gonorrhoeae μ μ μ
13 Total score (symptoms and laboratory findings) (A) (B) Outlier Max 75% file Average Median 25% file Min Day 0 EOT TOC Day 0 EOT TOC Fig.4.Changes in the total score (Comparison between new and formar scoring system). (A) New scoring system, White bar: Patients without a purulent discharge (n15), Black bar: Patients with a purulent discharge (n5), (B) Former scoring system EOT: End of treatment, TOC: Test of cure It was analyzed using data of the patients with intrauterine infection (n20) of the following clinical test. 10) Clinical study of levofloxacin 500 mg qd in the treatment of cervicitis with Chlamydia trachomatis and intrauterine infections: Jpn.J.Antibiotics. 2011: 64:
14 μ μ C. trachomatis
15 μ μ μ
日本化学療法学会雑誌第65巻第4号
 Mycoplasma pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae Key words Mycoplasma pneumoniae Mycoplasma pneumoniae M. pneumoniae I M. pneumoniae M. pneumoniae M. pneumoniae
Mycoplasma pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae Key words Mycoplasma pneumoniae Mycoplasma pneumoniae M. pneumoniae I M. pneumoniae M. pneumoniae M. pneumoniae
日本化学療法学会雑誌第61巻第4号
 μ μ μ μ μ μ Key words I β μ Sex Age (years) Height (cm) Evaluation items Table1.Characteristics of patients 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) male 12 8 7 27 female 2 1
μ μ μ μ μ μ Key words I β μ Sex Age (years) Height (cm) Evaluation items Table1.Characteristics of patients 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) male 12 8 7 27 female 2 1
日本化学療法学会雑誌第60巻第4号
 Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Key words β Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae μ μ S. pneumoniae H. influenzae S. pneumoniae
Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Key words β Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae μ μ S. pneumoniae H. influenzae S. pneumoniae
日本化学療法学会雑誌第56巻第1号
 β β β β β Streptococcus pneumoniaehaemophilus influenzaemoraxella catarrhalismycoplasma pneumoniaechlamydia pneumoniae β Key wordsβ mys nilc laci ngis Table. Assessmentschedule Parameters Patientcharacteristics
β β β β β Streptococcus pneumoniaehaemophilus influenzaemoraxella catarrhalismycoplasma pneumoniaechlamydia pneumoniae β Key wordsβ mys nilc laci ngis Table. Assessmentschedule Parameters Patientcharacteristics
日本化学療法学会雑誌第61巻第6号
 β Moraxella catarrhalis Escherichia coli Citrobacter Klebsiella pneumoniae Enterobacter cloacae Serratia marcescens Proteus Pseudomonas aeruginosa Acinetobacter Bacteroides fragilis β Haemophilus influenzae
β Moraxella catarrhalis Escherichia coli Citrobacter Klebsiella pneumoniae Enterobacter cloacae Serratia marcescens Proteus Pseudomonas aeruginosa Acinetobacter Bacteroides fragilis β Haemophilus influenzae
日本化学療法学会雑誌第59巻第5号
 Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
日本化学療法学会雑誌第57巻第6号
 Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae S. pneumoniae β β H. influenzae S. pneumoniae H. influenzae M. catarrhalis Key words Otalgia Fever Item Crying,Iritation,
Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae S. pneumoniae β β H. influenzae S. pneumoniae H. influenzae M. catarrhalis Key words Otalgia Fever Item Crying,Iritation,
CHEMOTHERAPY
 CHEMOTHERAPY VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin CHEMOTHERAPY AUG. 1993 VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin Table 1. Comparative in vitro activity of teicoplanin
CHEMOTHERAPY VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin CHEMOTHERAPY AUG. 1993 VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin Table 1. Comparative in vitro activity of teicoplanin
日本化学療法学会雑誌第57巻第5号
 in vitro Key words Streptococcus pneumoniae β Haemophilus influenzae Escherichia coli I Table. Antibacteriallevofloxacinactivityagainstclinicalisolates Organism Year Susceptibility(%)(Numberofstrains)
in vitro Key words Streptococcus pneumoniae β Haemophilus influenzae Escherichia coli I Table. Antibacteriallevofloxacinactivityagainstclinicalisolates Organism Year Susceptibility(%)(Numberofstrains)
Fig.2. Sensitivity distribution of clinical isolates of S. epidermidis (24 strains, 106 CFU/ml) Staphylococcus aureus Staphylococcus epider- midis Ent
 Fig.2. Sensitivity distribution of clinical isolates of S. epidermidis (24 strains, 106 CFU/ml) Staphylococcus aureus Staphylococcus epider- midis Enterococcus faecalis Klebsiella pneumoniae, Morganella
Fig.2. Sensitivity distribution of clinical isolates of S. epidermidis (24 strains, 106 CFU/ml) Staphylococcus aureus Staphylococcus epider- midis Enterococcus faecalis Klebsiella pneumoniae, Morganella
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study
 CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
366 12 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 Dec. 2012 1 8 DNA 2,3 16 12 20 171 2008 12 2010 11 2 3,558 4.44% 1.65% 1.17% 90% 9 Escherichia coli -
 Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 365 11 sita oxacin 1 1 1 1 1 1 2 2 3 3 1 1 1 2 3 2012 9 14 sita oxacin STFX 50 mg 10% 2008 1 2008 12 2010 11 2 STFX 1,452 91.4% 1,235/1,351 95.9% 466/486
Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 365 11 sita oxacin 1 1 1 1 1 1 2 2 3 3 1 1 1 2 3 2012 9 14 sita oxacin STFX 50 mg 10% 2008 1 2008 12 2010 11 2 STFX 1,452 91.4% 1,235/1,351 95.9% 466/486
日本化学療法学会雑誌第58巻第4号
 Escherichia coli Enterococcus faecalisstreptococcus agalactiae Klebsiella pneumoniae Staphylococcus epidermidis E. colie. faecalispseudomonas aeruginosa K. pneumoniae S. agalactiae E. coli E. coli μ p
Escherichia coli Enterococcus faecalisstreptococcus agalactiae Klebsiella pneumoniae Staphylococcus epidermidis E. colie. faecalispseudomonas aeruginosa K. pneumoniae S. agalactiae E. coli E. coli μ p
日本化学療法学会雑誌第64巻第4号
 β β Moraxella catarrhalisescherichia colicitrobacter Klebsiella pneumoniaeenterobacter cloacaeserratia marcescens Proteus Providencia Pseudomonas aeruginosaacinetobacter Bacteroides fragilis β β E. colik.
β β Moraxella catarrhalisescherichia colicitrobacter Klebsiella pneumoniaeenterobacter cloacaeserratia marcescens Proteus Providencia Pseudomonas aeruginosaacinetobacter Bacteroides fragilis β β E. colik.
CHEMOTHERAPY aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Prote
 aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Proteus mirabilis 3.13, Proteus vulgaris 1.56, Citrobacter freundii 0.39,
aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Proteus mirabilis 3.13, Proteus vulgaris 1.56, Citrobacter freundii 0.39,
日本化学療法学会雑誌第55巻第S-1号
 µ µ Key words Streptococcus pneu- moniae S. pneumoniae µ µ I γ H H C HN F F O N O CO H H C SO H H O Fig.. ChemicalstructureofGRNX. γ γ II Haemophilus influenzae Pseudomonas aeruginosa Streptococcus intermedius
µ µ Key words Streptococcus pneu- moniae S. pneumoniae µ µ I γ H H C HN F F O N O CO H H C SO H H O Fig.. ChemicalstructureofGRNX. γ γ II Haemophilus influenzae Pseudomonas aeruginosa Streptococcus intermedius
 CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
VOL.42 S-1
 CHEMOTHERAPY APR. 1994 VOL.42 S-1 CHEMOTHERAPY APR. 1994 Table 1. Criteria for evaluation of clinical efficacy by the Japanese Society of Oral and Maxillo-Facial Surgeons Grades of symptoms and numerical
CHEMOTHERAPY APR. 1994 VOL.42 S-1 CHEMOTHERAPY APR. 1994 Table 1. Criteria for evaluation of clinical efficacy by the Japanese Society of Oral and Maxillo-Facial Surgeons Grades of symptoms and numerical
VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity of gram nega
 1066 CHEMOTHERAPY MAR. 1975 Table 1 Sensitivity of standard strains VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity
1066 CHEMOTHERAPY MAR. 1975 Table 1 Sensitivity of standard strains VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity
日本化学療法学会雑誌第57巻第S-2号
 Key words β I Table. Observationandtestschedule Testschedule Observation/Tests Beforetreatment Endoftreatment 5 9days aftercompletion oftreatment 4 6weeks aftercompletion oftreatment Informedconsent Patientbackground
Key words β I Table. Observationandtestschedule Testschedule Observation/Tests Beforetreatment Endoftreatment 5 9days aftercompletion oftreatment 4 6weeks aftercompletion oftreatment Informedconsent Patientbackground
 600mg 600mg CTD 2 2.5 2.5 Page 3 2.5...7 2.5.1...7 2.5.2...27 2.5.3...28 2.5.4...42 2.5.5...55 2.5.6...79 2.5.7...97 2.5 Page 5 73 67 31 48 48A 102 104 105 106 ALP ALT(GPT) AST(GOT) AUC AUEC BID BUN
600mg 600mg CTD 2 2.5 2.5 Page 3 2.5...7 2.5.1...7 2.5.2...27 2.5.3...28 2.5.4...42 2.5.5...55 2.5.6...79 2.5.7...97 2.5 Page 5 73 67 31 48 48A 102 104 105 106 ALP ALT(GPT) AST(GOT) AUC AUEC BID BUN
CHEMOTHERAPY FEB Table 1. Activity of cefpirome and others against clinical isolates
 VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
400 46 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 Dec. 2012 LVFX 100 mg 3 / 7 150 mg 2 / 7 2 2006 2008 9 LVFX PK PD 2009 7 100 mg 1 3 500 mg 1 1 AUC/MIC
 Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
日本化学療法学会雑誌第54巻第S-1号
 β β Key words β Candida krusei Candida glabrata β I β Drugadministration Baselinecharacteristics Clinicalsymptom Mycologicaltests Plasmadrugconcn Adverseevents Laboratorytests ) -leadecg Clinicalphotograph
β β Key words β Candida krusei Candida glabrata β I β Drugadministration Baselinecharacteristics Clinicalsymptom Mycologicaltests Plasmadrugconcn Adverseevents Laboratorytests ) -leadecg Clinicalphotograph
Staphylococcus sp. K.pneumoniae P.mirabilis C.freundii E. cloacae Serratia sp. P. aeruginosa ml, Enterococcus avium >100ƒÊg/ml
 CHEMOTHERAPY SEPT. 1992 cefoperazone ceftazidime (CAZ), imipenem (IPM) Staphylococcus sp., Enterococcus (CPZ), faecalis, Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii, Enterobacter cloacae,
CHEMOTHERAPY SEPT. 1992 cefoperazone ceftazidime (CAZ), imipenem (IPM) Staphylococcus sp., Enterococcus (CPZ), faecalis, Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii, Enterobacter cloacae,
 1.Streptococcus pneumoniae, Streptococcus pyogenes JC-1,S.aureus Smith,methicillin (DMPPC)- susceptible S. aureus subsp. aureus (MSSA) TR101, DMPPC-resistant S. aureus subsp. aureus (MRSA) TR102, Staphylococcus
1.Streptococcus pneumoniae, Streptococcus pyogenes JC-1,S.aureus Smith,methicillin (DMPPC)- susceptible S. aureus subsp. aureus (MSSA) TR101, DMPPC-resistant S. aureus subsp. aureus (MRSA) TR102, Staphylococcus
CHEMOTHERAPY JUN Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter cloacae 27, Proteus rettgeri 7, Proteus inconstans 20, Proteus
 VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
日本化学療法学会雑誌第56巻第S-1号
 Key words Streptococcus pneumoniae µ µ H F Cl H H N N H N F COOH O Fig.1. Sitafloxacinstructure. I γ S. pneumoniae Haemophilus influenzae Table1. Observationandtestschedule Observation/Tests Informedconsent
Key words Streptococcus pneumoniae µ µ H F Cl H H N N H N F COOH O Fig.1. Sitafloxacinstructure. I γ S. pneumoniae Haemophilus influenzae Table1. Observationandtestschedule Observation/Tests Informedconsent
Fig. 1 Chemical structure of norfioxacin (AM-715)
 Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
MIC MIC...
 50 mg 10% 2.7.36 2.7.36 2.7.36... 1 1.6... 1 2.6... 3 3.6... 5 3.16... 5 3.26... 12 3.36... 16 4.6... 17 5.6... 19 6.6... 20 2.7.3.3.16-1 MIC... 9 2.7.3.3.16-2 MIC... 10 2.7.3.3.16-3 MIC E. coli... 11
50 mg 10% 2.7.36 2.7.36 2.7.36... 1 1.6... 1 2.6... 3 3.6... 5 3.16... 5 3.26... 12 3.36... 16 4.6... 17 5.6... 19 6.6... 20 2.7.3.3.16-1 MIC... 9 2.7.3.3.16-2 MIC... 10 2.7.3.3.16-3 MIC E. coli... 11
Fig.1 Chemical structure of BAY o 9867
 Fig.1 Chemical structure of BAY o 9867 CHEMOTHERAPY 43 Table 3 Antibacterial spectrum of gram negative bacteria Medium:Heart infusion agar (Nissui) Method:Agar dilution (Streak) CHEMOTHERAPY DEC 1985
Fig.1 Chemical structure of BAY o 9867 CHEMOTHERAPY 43 Table 3 Antibacterial spectrum of gram negative bacteria Medium:Heart infusion agar (Nissui) Method:Agar dilution (Streak) CHEMOTHERAPY DEC 1985
日本化学療法学会雑誌第53巻第S-3号
 moxifloxacin in vitro moxifloxacin in vitro 17 9 6 17 11 21 moxifloxacinmflx in vitro cefdinir CFDNclavulanic acidamoxicillincvaampcclarithromycincamclindamycincldm levofloxacinlvfx 1MFLX Clostridium clostridiiformeclostridium
moxifloxacin in vitro moxifloxacin in vitro 17 9 6 17 11 21 moxifloxacinmflx in vitro cefdinir CFDNclavulanic acidamoxicillincvaampcclarithromycincamclindamycincldm levofloxacinlvfx 1MFLX Clostridium clostridiiformeclostridium
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates
 Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
epidermidis, Enterococcus faecalis, Enterococcus Klebsiella pneumoniae, Proteus mirabilis, indolepositive Proteus spp., Enterobacter spp., Serratia
 epidermidis, Enterococcus faecalis, Enterococcus Klebsiella pneumoniae, Proteus mirabilis, indolepositive Proteus spp., Enterobacter spp., Serratia Table 3. Overall clinical efficacy of cefozopran in
epidermidis, Enterococcus faecalis, Enterococcus Klebsiella pneumoniae, Proteus mirabilis, indolepositive Proteus spp., Enterobacter spp., Serratia Table 3. Overall clinical efficacy of cefozopran in
VOL.32 S-7 CHEMOTHERAPY Table 1 MIC of standard strains of CTRX Fig. 2 Cumulative curves of MIC S. aureus (26 strains )
 CHEMOTHERAPY OCT. 1984 Fig. I Chemical structure of CTRX VOL.32 S-7 CHEMOTHERAPY Table 1 MIC of standard strains of CTRX Fig. 2 Cumulative curves of MIC S. aureus (26 strains ) CHEMOTHERAPY Fig. 3 Cumulative
CHEMOTHERAPY OCT. 1984 Fig. I Chemical structure of CTRX VOL.32 S-7 CHEMOTHERAPY Table 1 MIC of standard strains of CTRX Fig. 2 Cumulative curves of MIC S. aureus (26 strains ) CHEMOTHERAPY Fig. 3 Cumulative
Fig. 1 Chemical structure of DL-8280
 Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
2.7 臨床概要
 2.7 臨床概要 A/G Al-P ALT AST AT- AUC AZM BIPM BP BUN CEZ CFPN-PI CFU CLcr Cmax CNS COPD CPFX CRP CS CYP CVA DBT DHP-I DIC DRPM DRPM-DC FAS γ-gtp GCP GNB GNF-GNR GPB HAM - Staphylococcus C P450 -I Full Analysis
2.7 臨床概要 A/G Al-P ALT AST AT- AUC AZM BIPM BP BUN CEZ CFPN-PI CFU CLcr Cmax CNS COPD CPFX CRP CS CYP CVA DBT DHP-I DIC DRPM DRPM-DC FAS γ-gtp GCP GNB GNF-GNR GPB HAM - Staphylococcus C P450 -I Full Analysis
988 CHEMOTHERAPY NOV. 1971
 988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
日本化学療法学会雑誌第59巻第6号
 Key words Survey forms collected: 32,200 Patients subject to safety analysis: 29,880 Excluded from safety analysis: 2,320 Reasons: Administration outside contract period: 6 Registered 8 days after the
Key words Survey forms collected: 32,200 Patients subject to safety analysis: 29,880 Excluded from safety analysis: 2,320 Reasons: Administration outside contract period: 6 Registered 8 days after the
 1) Kojima H: Culture of ejaculate for Neisseria gonorrhoeae, 1st Serually Transmitted Diseases World Congress, San Juan, 11. 19, 1981. 9) Kojima H, Mori C: Clinical studies on Conococcal and Chlamydial
1) Kojima H: Culture of ejaculate for Neisseria gonorrhoeae, 1st Serually Transmitted Diseases World Congress, San Juan, 11. 19, 1981. 9) Kojima H, Mori C: Clinical studies on Conococcal and Chlamydial
VOL. 40 S- 1 Table 1. Susceptibility of methicillin-resistant Staphylococcus aureus to meropenem Table 2. Coagulase typing of methicillin-resistant St
 CHEMOTHERAPY VOL. 40 S- 1 Table 1. Susceptibility of methicillin-resistant Staphylococcus aureus to meropenem Table 2. Coagulase typing of methicillin-resistant Staphylococcus aureus CHEMOTHERAPY Table
CHEMOTHERAPY VOL. 40 S- 1 Table 1. Susceptibility of methicillin-resistant Staphylococcus aureus to meropenem Table 2. Coagulase typing of methicillin-resistant Staphylococcus aureus CHEMOTHERAPY Table
 Table 1. Antibacterial activity of cefdinir, cefixime, cefteram, cefuroxime, cefaclor and amoxicillin against standard strains Inoculum size: 108 cells/ml CFDN: cefdinir, CFIX: cefixime, CFTM: cefteram,
Table 1. Antibacterial activity of cefdinir, cefixime, cefteram, cefuroxime, cefaclor and amoxicillin against standard strains Inoculum size: 108 cells/ml CFDN: cefdinir, CFIX: cefixime, CFTM: cefteram,
 Table 1 Sensitivity distribution of clinical isolates 1. Escherichia coli Inoculum size: 106cells/ml 2. Klebsiella pneumoniae 3. Enterobacter cloacae 4. Serratia marcescens Inoculum size: 106cells/nil
Table 1 Sensitivity distribution of clinical isolates 1. Escherichia coli Inoculum size: 106cells/ml 2. Klebsiella pneumoniae 3. Enterobacter cloacae 4. Serratia marcescens Inoculum size: 106cells/nil
CHEMOTHERAPY DEC (NFLX), ofloxacin (OFLX), ciprofloxacin (CPFX) Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecali
 CHEMOTHERAPY DEC. 1988 (NFLX), ofloxacin (OFLX), ciprofloxacin (CPFX) Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis Pseudomonas aeruginosa, Serratia ma- Fig. 1. Chemical
CHEMOTHERAPY DEC. 1988 (NFLX), ofloxacin (OFLX), ciprofloxacin (CPFX) Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis Pseudomonas aeruginosa, Serratia ma- Fig. 1. Chemical
CHEMOTHERAPY DEC Table 1 Antibacterial spectra of T-1982, CTT, CMZ, CTX, CPZ and CEZ 106 CFU/ml Note: P; Peptococcus, S; Streptococcus, G; Gaffk
 VOL. 30 S-3 CHEMOTHERAPY imeumoniae, Serratia marcescens, Proteus sp, CHEMOTHERAPY DEC. 1982 Table 1 Antibacterial spectra of T-1982, CTT, CMZ, CTX, CPZ and CEZ 106 CFU/ml Note: P; Peptococcus, S; Streptococcus,
VOL. 30 S-3 CHEMOTHERAPY imeumoniae, Serratia marcescens, Proteus sp, CHEMOTHERAPY DEC. 1982 Table 1 Antibacterial spectra of T-1982, CTT, CMZ, CTX, CPZ and CEZ 106 CFU/ml Note: P; Peptococcus, S; Streptococcus,
日本化学療法学会雑誌第51巻第2号
 piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
THE JAPANESE JOURNAL OF ANTIBIOTICS 68 3 June 2015 Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis % 2 S. pneumon
 June 2015 THE JAPANESE JOURNAL OF ANTIBIOTICS 68 3 189 49 1 : 14 1 2 2 3 1 2 3 2015 4 3 1 : 14 CVA/AMPC 1 : 14 27 CVA/AMPC 1 : 14 88.5% Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis
June 2015 THE JAPANESE JOURNAL OF ANTIBIOTICS 68 3 189 49 1 : 14 1 2 2 3 1 2 3 2015 4 3 1 : 14 CVA/AMPC 1 : 14 27 CVA/AMPC 1 : 14 88.5% Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis
untitled
 Seminal paper Anhalt JP Pioneering paper Claydon MA Kishnamurthy T Enterobacter B. cereus Routine identification paper Eigner U Seng P Cherkaoui A Saureus S. Saureus* S. Vagococcus Haemophilus Mycobacterium
Seminal paper Anhalt JP Pioneering paper Claydon MA Kishnamurthy T Enterobacter B. cereus Routine identification paper Eigner U Seng P Cherkaoui A Saureus S. Saureus* S. Vagococcus Haemophilus Mycobacterium
CHEMOTHERAPY Methicillin-resistant S.aureus(MRSA) coccus epidermidis 105 Streptococcus pyogenes E.faecali senterococcus avium Enterococcus faecium Str
 cefaclor(ccl),cefuroxime(cxm),cefixime (CFIX),cefteram(CFTM),cefdinir(CFDN) pneumoniae,streptococcus pyogenes Moraxella catarrhalis,haemophilus influenzae,escherichia coli, Klebsiella pneumoniae,proteus
cefaclor(ccl),cefuroxime(cxm),cefixime (CFIX),cefteram(CFTM),cefdinir(CFDN) pneumoniae,streptococcus pyogenes Moraxella catarrhalis,haemophilus influenzae,escherichia coli, Klebsiella pneumoniae,proteus
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S.
 Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone
 Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone (inoculum size= 106 CFU/ml) (Ĉ-lactamase producer : 2 strains) Fig. 1. Sensitivity distribution of
Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone (inoculum size= 106 CFU/ml) (Ĉ-lactamase producer : 2 strains) Fig. 1. Sensitivity distribution of
THE JAPANESE JOURNAL OF ANTIBIOTICS 48-8 Enterococcus avium 5Š, Corynebacterium xerosis 10Š, Corynebacterium pseudodiphtheriticum 10Š, Corynebacterium
 THE JAPANESE JOURNAL OF ANTIBIOTICS 48-8 Enterobacter spp., Serratia spp., Burkholderia cepacia, Flavobacterium spp., Alcaligenes spp. THE JAPANESE JOURNAL OF ANTIBIOTICS 48-8 Enterococcus avium 5Š, Corynebacterium
THE JAPANESE JOURNAL OF ANTIBIOTICS 48-8 Enterobacter spp., Serratia spp., Burkholderia cepacia, Flavobacterium spp., Alcaligenes spp. THE JAPANESE JOURNAL OF ANTIBIOTICS 48-8 Enterococcus avium 5Š, Corynebacterium
 Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, F
 VOL.43 S-1 coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, Flavobacter- Table 1. Concentration of grepafloxacin
VOL.43 S-1 coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, Flavobacter- Table 1. Concentration of grepafloxacin
 CHEMOTHERAPY APRIL 1992 VOL. 40 S- 1 Table 1-1. Comparative in vitro activity of meropenem against clinical isolates CNS: coagulase-negative staphylococci CHEMOTHERAPY APRIL 1992 Table 1-2. Comparative
CHEMOTHERAPY APRIL 1992 VOL. 40 S- 1 Table 1-1. Comparative in vitro activity of meropenem against clinical isolates CNS: coagulase-negative staphylococci CHEMOTHERAPY APRIL 1992 Table 1-2. Comparative
Clostridium difficile ciprofloxacin, ofloxacin, norfloxacin Bifidobacterium Lactobacillus Lactobacillus Bacteroides fragilis B. fragilis C. difficile
 Clostridium difficile ciprofloxacin, ofloxacin, norfloxacin Bifidobacterium Lactobacillus Lactobacillus Bacteroides fragilis B. fragilis C. difficile Key words: temafloxacin, TA-167, Bacteroides fragilis,
Clostridium difficile ciprofloxacin, ofloxacin, norfloxacin Bifidobacterium Lactobacillus Lactobacillus Bacteroides fragilis B. fragilis C. difficile Key words: temafloxacin, TA-167, Bacteroides fragilis,
VOL.35 S-2 CHEMOTHERAPY Table 1 Sex and age distribution Table 2 Applications of treatment with carumonam Table 3 Concentration of carumonam in human
 CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
日本化学療法学会雑誌第57巻第S-2号
 µ µ Key words µ µ µ I Table1. Subjectprofilesin(A)singleand(B)multiple-dosestudies (A)Single-dosestudy Age (yr) Height (cm) Bodyweight (kg) BMI (kg/m ) Ccr(Cockcroft) (ml/min) Ethnicity dose(mg) n 5 5.1±.,3
µ µ Key words µ µ µ I Table1. Subjectprofilesin(A)singleand(B)multiple-dosestudies (A)Single-dosestudy Age (yr) Height (cm) Bodyweight (kg) BMI (kg/m ) Ccr(Cockcroft) (ml/min) Ethnicity dose(mg) n 5 5.1±.,3
Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-
 Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl) oxy]-14-ethyl-12,
Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl) oxy]-14-ethyl-12,
Fig. 1 Chemical structure of KW-1070
 Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
CHEMOTHERAPY
 VOL.40 S-1 coagulase negative Staphylococcus sp, methicillin- resistant Staphylococcus aureus (MRSA), Enterococcus faecalis, Escherichia coli, Citrobacter freundii, Klebsiella pneumoniae, Enterobacter
VOL.40 S-1 coagulase negative Staphylococcus sp, methicillin- resistant Staphylococcus aureus (MRSA), Enterococcus faecalis, Escherichia coli, Citrobacter freundii, Klebsiella pneumoniae, Enterobacter
VOL.39 S-3
 VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
日本化学療法学会雑誌第57巻第1号
 In vitro Streptococcus pneumoniae Escherichia coli in vitro Streptococcus pneumoniae Escherichia coli µs. pneumoniae E. coli µ Key words in vitrostreptococcus pneumoniae Escherichia coli Escherichia coli
In vitro Streptococcus pneumoniae Escherichia coli in vitro Streptococcus pneumoniae Escherichia coli µs. pneumoniae E. coli µ Key words in vitrostreptococcus pneumoniae Escherichia coli Escherichia coli
8 The Bulletin of Meiji University of Integrative Medicine API II 61 ASO X 11 7 X-4 6 X m 5 X-2 4 X 3 9 X 11 7 API 0.84 ASO X 1 1 MR-angio
 7-14 2010 1 1 1 2 1 1 1 2 Fontaine II ASO61 3 API ASO ASO ASO API API KKKKKKKKKK ASO Fontaine II API Received April 14, 2009; Accepted July 16, 2009 I arteriosclerosis obliterans: ASO ASO 50 70 1,2 Fontaine
7-14 2010 1 1 1 2 1 1 1 2 Fontaine II ASO61 3 API ASO ASO ASO API API KKKKKKKKKK ASO Fontaine II API Received April 14, 2009; Accepted July 16, 2009 I arteriosclerosis obliterans: ASO ASO 50 70 1,2 Fontaine
第65回日本化学療法学会東日本支部総会 抄録
 鍵 76 Clostridioides difficile C. difficile 77 γ γ Mycobacterium avium 78 79 Clostridium difficile Treponema pallidum 80 81 82 in vitro in vitro 83 鍵 Treponema pallidum 84 Chlamydia trachomatis Mycoplasma
鍵 76 Clostridioides difficile C. difficile 77 γ γ Mycobacterium avium 78 79 Clostridium difficile Treponema pallidum 80 81 82 in vitro in vitro 83 鍵 Treponema pallidum 84 Chlamydia trachomatis Mycoplasma
VOL.42 S-1 methicillin-susceptible Staphylococcus aureus (MSSA), Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Klebsiella pneum
 VOL.42 S-1 methicillin-susceptible Staphylococcus aureus (MSSA), Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae methicillin-resistant Staphylococcus
VOL.42 S-1 methicillin-susceptible Staphylococcus aureus (MSSA), Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae methicillin-resistant Staphylococcus
CHEMOTHERAPY SEPT. 1991
 CHEMOTHERAPY SEPT. 1991 VOL. 39 S-3 Table 1. Background of characteristics and allocation of 9 healthy male volunteers in a single-dose study on betamipron Table 2. Background of characteristics and allocation
CHEMOTHERAPY SEPT. 1991 VOL. 39 S-3 Table 1. Background of characteristics and allocation of 9 healthy male volunteers in a single-dose study on betamipron Table 2. Background of characteristics and allocation
1272 CHEMOTHERAPY MAR. 1975
 1272 CHEMOTHERAPY MAR. 1975 VOL. 23 NO. 3 CHEMOTHERAPY 1273 Fig. 2 Minimal inhibitory concentration of aminoglycosides against 50 strains of Klebsiella Fig. 1 Minimal inhibitory concentration of aminoglycosides
1272 CHEMOTHERAPY MAR. 1975 VOL. 23 NO. 3 CHEMOTHERAPY 1273 Fig. 2 Minimal inhibitory concentration of aminoglycosides against 50 strains of Klebsiella Fig. 1 Minimal inhibitory concentration of aminoglycosides
日本化学療法学会雑誌第56巻第3号
 β Key words Candida albicans albicans Candida C. albicans Candida glabrata Candida krusei albicans Candida C. glabrata C. albicans albicans Candida β in vitro in vivo C. glabrata C. krusei I β γ µ Candida
β Key words Candida albicans albicans Candida C. albicans Candida glabrata Candida krusei albicans Candida C. glabrata C. albicans albicans Candida β in vitro in vivo C. glabrata C. krusei I β γ µ Candida
Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII (45)
 Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2305(45) 2306(46) THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 Dec. Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2307(47) 2308(48) THE JAPANESE
Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2305(45) 2306(46) THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 Dec. Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2307(47) 2308(48) THE JAPANESE
R06_01
 Staphylococcus aureus (MSSA) PCG (N=118,334) 57,369 (48.5%) 判定不能 :3 (0.0%) 60,962 (51.5%) CEZ (N=143,723) I:42 (0.0%) 143,635 (99.9%) R:46 (0.0%) CVA/AMPC (N=19,281) R:14 (0.1%) 19,265 (99.9%) 判定不能 :2
Staphylococcus aureus (MSSA) PCG (N=118,334) 57,369 (48.5%) 判定不能 :3 (0.0%) 60,962 (51.5%) CEZ (N=143,723) I:42 (0.0%) 143,635 (99.9%) R:46 (0.0%) CVA/AMPC (N=19,281) R:14 (0.1%) 19,265 (99.9%) 判定不能 :2
CHEMOTHERAPY NOV S. aureus, S. epidermidis, E. coli, K. pgeumoniae, E. cloacae, S. marcescens, P. mirabilis, Proteus, P. aeruginosa Inoculum siz
 VOL.33 S-5 CHEMOTHERAPY 381 Fig. 1 Chemical structure of HAPA-B Chemical name 1-N-[(2S)-3-Amino-2-hydroxypropiony1]-4-0-(6-amino- 6-deoxy-a-D-glucopyranosyl)-6-013-deoxy-4-C-methyl- 3-(methylamino)-ƒÀ-L-arabinopyranosyl]-2-deoxystreptamine
VOL.33 S-5 CHEMOTHERAPY 381 Fig. 1 Chemical structure of HAPA-B Chemical name 1-N-[(2S)-3-Amino-2-hydroxypropiony1]-4-0-(6-amino- 6-deoxy-a-D-glucopyranosyl)-6-013-deoxy-4-C-methyl- 3-(methylamino)-ƒÀ-L-arabinopyranosyl]-2-deoxystreptamine
Feb THE JAPANESE JOURNAL OF ANTIBIOTICS Tebipenem pivoxil 1 1, Meiji Seika 2 Meiji Seika G 3 Meiji Seika Tebipen
 Feb. 2016 THE JAPANESE JOURNAL OF ANTIBIOTICS 69 1 53 53 Tebipenem pivoxil 1 1, 3 2 2 1 1 Meiji Seika 2 Meiji Seika G 3 Meiji Seika 2015 12 15 Tebipenem pivoxil 10% 2010 4 2013 3 3,547 3,540 3,540 3,331
Feb. 2016 THE JAPANESE JOURNAL OF ANTIBIOTICS 69 1 53 53 Tebipenem pivoxil 1 1, 3 2 2 1 1 Meiji Seika 2 Meiji Seika G 3 Meiji Seika 2015 12 15 Tebipenem pivoxil 10% 2010 4 2013 3 3,547 3,540 3,540 3,331
208 ( 2 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 63 _ 3 June 2010 Cefditoren pivoxil (CDTR-PI) MS MS 10%
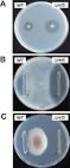 207 ( 1 ) Cefditoren pivoxil G 2010 2 2 2006 3 Cefditoren pivoxil CDTR-PI MS 10% CDTR-PI 305 2,144 2,006 1,958 1.79% 36 2,006 26 (1.30%) CDTR-PI CDTR-PI (9 mg/kg/day) 1.5 2 (2.70%) 2 (1.92%) 93.5% 1,831
207 ( 1 ) Cefditoren pivoxil G 2010 2 2 2006 3 Cefditoren pivoxil CDTR-PI MS 10% CDTR-PI 305 2,144 2,006 1,958 1.79% 36 2,006 26 (1.30%) CDTR-PI CDTR-PI (9 mg/kg/day) 1.5 2 (2.70%) 2 (1.92%) 93.5% 1,831
CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels of mezlocillin
 CHEMOTHERAPY Fig. 2 Urinary excretion of mezlocillin Fig. 1 Blood levels of mezlocillin CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels
CHEMOTHERAPY Fig. 2 Urinary excretion of mezlocillin Fig. 1 Blood levels of mezlocillin CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels
Key words: bacterial meningitis, Haemophilus influenzae type b, Streptococcus pneumoniae, rapid diagnosis, childhood
 Key words: bacterial meningitis, Haemophilus influenzae type b, Streptococcus pneumoniae, rapid diagnosis, childhood Fig.1 Distribution of the cases with bacterial meningitis by age and pathogens Chiba
Key words: bacterial meningitis, Haemophilus influenzae type b, Streptococcus pneumoniae, rapid diagnosis, childhood Fig.1 Distribution of the cases with bacterial meningitis by age and pathogens Chiba
Table 1 Classification of female patients with vesical irritating symptom by their signs : Urinary pain with or without other vesical irritability. s
 Table 1 Classification of female patients with vesical irritating symptom by their signs : Urinary pain with or without other vesical irritability. s Vesical irritability without urinary Pain. Pyuria 10/
Table 1 Classification of female patients with vesical irritating symptom by their signs : Urinary pain with or without other vesical irritability. s Vesical irritability without urinary Pain. Pyuria 10/
 Table 1.Quality control of MICs for reference strains Table 2.Antimicrobial activity of gatifloxacin against aerobic bacteria Table 4.Antimicrobial activity of gatifloxacin and other quinolones against
Table 1.Quality control of MICs for reference strains Table 2.Antimicrobial activity of gatifloxacin against aerobic bacteria Table 4.Antimicrobial activity of gatifloxacin and other quinolones against
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, Sc
 A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST
 Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
HPM_442_F_TgCHG_1128
 3M TM Chlorhexidine Gluconate IV Securement Dressings Tegaderm TM CHG Support CRBSI Prevention TegadermTM CHG Dressing 2w/w% 60% N. Safdar, Intensive Care Med 2004 30 62-7 Guideline Centers for Disease
3M TM Chlorhexidine Gluconate IV Securement Dressings Tegaderm TM CHG Support CRBSI Prevention TegadermTM CHG Dressing 2w/w% 60% N. Safdar, Intensive Care Med 2004 30 62-7 Guideline Centers for Disease
 Osamu NEMOTO, M.D. Clinical and Bacteriological Research of Sucrose/ Povidone-Iodine Ointment (U-PASTA kowa) for Pressure Sores and Skin Ulcers Osamu Nemoto Department of Dermatology,Tonan Hosptal
Osamu NEMOTO, M.D. Clinical and Bacteriological Research of Sucrose/ Povidone-Iodine Ointment (U-PASTA kowa) for Pressure Sores and Skin Ulcers Osamu Nemoto Department of Dermatology,Tonan Hosptal
Aug THE JAPANESE JOURNAL OF ANTIBIOTICS (9) 2007 NTT JA
 Aug. 2009 THE JAPANESE JOURNAL OF ANTIBIOTICS 62 44 277 (9) 2007 NTT JA 278 (10) THE JAPANESE JOURNAL OF ANTIBIOTICS 62 _ 4 Aug. 2009 2009 4 10 1982 7 2007 2007 4 2008 3 1 229 181 (79.0%) 683 24 395 288
Aug. 2009 THE JAPANESE JOURNAL OF ANTIBIOTICS 62 44 277 (9) 2007 NTT JA 278 (10) THE JAPANESE JOURNAL OF ANTIBIOTICS 62 _ 4 Aug. 2009 2009 4 10 1982 7 2007 2007 4 2008 3 1 229 181 (79.0%) 683 24 395 288
Streptococcus pneumoniae,streptococcus pyogenes,streptococcus agalactiae,neisseria gonorrhoeae,h.influenzae,moraxella subgenus Branhamella catarrharis
 Streptococcus pneumoniae,streptococcus pyogenes,streptococcus agalactiae,neisseria gonorrhoeae,h.influenzae,moraxella subgenus Branhamella catarrharis, E.coil,Klebsiella pneumoniae,klebsiella oxytoca,proteus
Streptococcus pneumoniae,streptococcus pyogenes,streptococcus agalactiae,neisseria gonorrhoeae,h.influenzae,moraxella subgenus Branhamella catarrharis, E.coil,Klebsiella pneumoniae,klebsiella oxytoca,proteus
日本化学療法学会雑誌第58巻第2号
 Key words β Candida Table1. MCFGCPAstudygroup Investigator (representative) YoshitsuguMiyazaki NaoyukiMiyashita RyoichiAmitani KenjiOgawa AtsuyukiKurashima ToshiroKiguchi MichiakiMishima YuichiInoue HiroshiSaito
Key words β Candida Table1. MCFGCPAstudygroup Investigator (representative) YoshitsuguMiyazaki NaoyukiMiyashita RyoichiAmitani KenjiOgawa AtsuyukiKurashima ToshiroKiguchi MichiakiMishima YuichiInoue HiroshiSaito
日本化学療法学会雑誌第57巻第4号
 Streptococcus pneumoniae Haemophilus influenzae β β Key words I β Enterococcus faecium Pseudomonas aeruginosa Streptococcus pneumoniae S. pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Table.
Streptococcus pneumoniae Haemophilus influenzae β β Key words I β Enterococcus faecium Pseudomonas aeruginosa Streptococcus pneumoniae S. pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Table.
CHEMOTHERAPY
 CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of Sulbactam/CPZ against standard strains MIC mg/ml Inoculum size 106 CFU/ml * Sulbactam/CPZ= 1: 1 ** Concentration of Sulbactam+ CPZ CHEMOTHERAPY
CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of Sulbactam/CPZ against standard strains MIC mg/ml Inoculum size 106 CFU/ml * Sulbactam/CPZ= 1: 1 ** Concentration of Sulbactam+ CPZ CHEMOTHERAPY
CHEMOTHERAPY APR. 1982
 VOL.30 S-1 CHEMOTHERAPY Table 1 Dose of CTT and subjects i. v.: Intravenous bolus injection d. i. v.: Intravenous drip infusion i. m.: Intramuscular injection Fig. 1 Schedule for examination of CTT, 1.0g
VOL.30 S-1 CHEMOTHERAPY Table 1 Dose of CTT and subjects i. v.: Intravenous bolus injection d. i. v.: Intravenous drip infusion i. m.: Intramuscular injection Fig. 1 Schedule for examination of CTT, 1.0g
VOL. 23 NO. 3 CHEMOTHERAPY 1379 Table 1 Susceptibility of clinical isolated strains to Tobramycin
 VOL. 23 NO. 3 CHEMOTHERAPY 1379 Table 1 Susceptibility of clinical isolated strains to Tobramycin 1380 CHEMOTHERAPY MAR. 1975 Table 2 Susceptibility of isolated Pseudomonas aeruginosa to various antibiotics
VOL. 23 NO. 3 CHEMOTHERAPY 1379 Table 1 Susceptibility of clinical isolated strains to Tobramycin 1380 CHEMOTHERAPY MAR. 1975 Table 2 Susceptibility of isolated Pseudomonas aeruginosa to various antibiotics
CHEMOTHERAPY APRIL 1992 Acinetobacter calcoaceticus Staphylococcus aureus, Escherichia coli P. aeruginosa E. eoli, Klebsiella pneumoniae Serratia marc
 APRIL 1992 Acinetobacter calcoaceticus Staphylococcus aureus, Escherichia coli P. aeruginosa E. eoli, Klebsiella pneumoniae Serratia marcescens P. aeruginosa P. aeruginosa Streptococcus pyogenes Streptococcus
APRIL 1992 Acinetobacter calcoaceticus Staphylococcus aureus, Escherichia coli P. aeruginosa E. eoli, Klebsiella pneumoniae Serratia marcescens P. aeruginosa P. aeruginosa Streptococcus pyogenes Streptococcus
内科96巻3号★/NAI3‐1(第22回試験問題)
 µ µ α µ µ µ µ µ µ β β α γ µ Enterococcus faecalis Escherichia coli Legionella pneumophila Pseudomonas aeruginosa Streptococcus viridans α β 正解表正解記号問題 No. 正解記号問題 No. e(4.5) 26 e 1 a(1.2) 27 a 2
µ µ α µ µ µ µ µ µ β β α γ µ Enterococcus faecalis Escherichia coli Legionella pneumophila Pseudomonas aeruginosa Streptococcus viridans α β 正解表正解記号問題 No. 正解記号問題 No. e(4.5) 26 e 1 a(1.2) 27 a 2
CHEMOTHERAPY
 CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of BRL 28500 against standard strains of bacteria Fig, 1 Sensitivity distribution of ABPC-resistant E. coli isolated from urinary tract Fig. 2 Sensitivity
CHEMOTHERAPY CHEMOTHERAPY Table 1 Antibacterial activity of BRL 28500 against standard strains of bacteria Fig, 1 Sensitivity distribution of ABPC-resistant E. coli isolated from urinary tract Fig. 2 Sensitivity
 Table 1 Survival rates of infected mice given antibiotic doses producing peak serum a) S. aurcus Smith Challenge dose :7 ~10 (5% mucin) CFU/mouse. LD50: 1 ~103 (5% mucin) CFU/mouse. Table 2 Survival rates
Table 1 Survival rates of infected mice given antibiotic doses producing peak serum a) S. aurcus Smith Challenge dose :7 ~10 (5% mucin) CFU/mouse. LD50: 1 ~103 (5% mucin) CFU/mouse. Table 2 Survival rates
Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date URL
 Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date 1958-01 URL http://hdl.handle.net/2433/111559 Right Type Departmental Bulletin
Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date 1958-01 URL http://hdl.handle.net/2433/111559 Right Type Departmental Bulletin
Rinku General Medical Center
 Rinku General Medical Center 4860 100 1620 Ann Intern Med. 1966; 64: 328 40 CHOP 63 1 83 J Clin Oncol. 1998; 16: 20065-69 Febrile Neutropenia IDSA(Infectious Diseases Society of America) 2002 Guidelines
Rinku General Medical Center 4860 100 1620 Ann Intern Med. 1966; 64: 328 40 CHOP 63 1 83 J Clin Oncol. 1998; 16: 20065-69 Febrile Neutropenia IDSA(Infectious Diseases Society of America) 2002 Guidelines
CHEMOTHERAPY Fig. 1 Chemical structure of CXM-AX
 Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
 Table 1. Influence of urine ph on MBCs of new quinolones against Escherichia coli NIHJ JC-2 and Pseudomonas aeruginosa 18S; MBCs in urine were compared with those in Miieller-Hinton broth. Table 2. Influence
Table 1. Influence of urine ph on MBCs of new quinolones against Escherichia coli NIHJ JC-2 and Pseudomonas aeruginosa 18S; MBCs in urine were compared with those in Miieller-Hinton broth. Table 2. Influence
 A comparison of abdominal versus vaginal hysterectomy for leiomyoma and adenomyosis Kenji ARAHORI, Hisasi KATAYAMA, Suminori NIOKA Department of Obstetrics and Gnecology, National Maizuru Hospital,Kyoto,
A comparison of abdominal versus vaginal hysterectomy for leiomyoma and adenomyosis Kenji ARAHORI, Hisasi KATAYAMA, Suminori NIOKA Department of Obstetrics and Gnecology, National Maizuru Hospital,Kyoto,
pneumoniae 30, C. freundii 32, E. aerogenes 27, E. cloacae 32, P. mirabilis 31, P. vulgaris 34, M. morganii 32, S. marcescens 31, H. influenzae 27, P.
 pneumoniae 30, C. freundii 32, E. aerogenes 27, E. cloacae 32, P. mirabilis 31, P. vulgaris 34, M. morganii 32, S. marcescens 31, H. influenzae 27, P. aeruginosa 30, P. maltophilia pyogenes 32, Escherichia
pneumoniae 30, C. freundii 32, E. aerogenes 27, E. cloacae 32, P. mirabilis 31, P. vulgaris 34, M. morganii 32, S. marcescens 31, H. influenzae 27, P. aeruginosa 30, P. maltophilia pyogenes 32, Escherichia
Apr THE JAPANESE JOURNAL OF ANTIBIOTICS ( 13 ) 2008 NTT
 Apr. 2010 THE JAPANESE JOURNAL OF ANTIBIOTICS 63 12 105 ( 13 ) 2008 NTT 106 ( 14 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 63 _ 2 Apr. 2010 JA 2010 1 27 1982 7 2008 2008 4 2009 3 1 215 173 (80.5%) 694 18 357
Apr. 2010 THE JAPANESE JOURNAL OF ANTIBIOTICS 63 12 105 ( 13 ) 2008 NTT 106 ( 14 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 63 _ 2 Apr. 2010 JA 2010 1 27 1982 7 2008 2008 4 2009 3 1 215 173 (80.5%) 694 18 357
