サムチレール内用懸濁液 15% に関する資料 本資料に記載された情報に係る権利及び内容の責任はグラクソ スミスクライン株式会社に帰属するものであり 当該情報を適正使用以外の営利目的に利用することはできません グラクソ スミスクライン株式会社
|
|
|
- あいと むこやま
- 5 years ago
- Views:
Transcription
1 サムチレール内用懸濁液 15% に関する資料 本資料に記載された情報に係る権利及び内容の責任はグラクソ スミスクライン株式会社に帰属するものであり 当該情報を適正使用以外の営利目的に利用することはできません グラクソ スミスクライン株式会社
2 1.4. 特許状況 ( : ) ( : ) p. 1
3 1.5. 起原または発見の経緯および開発の経緯アトバコン ( 以下 本剤 ) は 社 ( 現 GlaxoSmithKline 社 ) により開発された抗ニューモシスチス活性を有するユビキノン類似体 (hydroxy-1,4-naphthoquinone) である 2011 年 2 月現在 米国 カナダ フランス 英国 ドイツを含む世界 21 ヵ国でニューモシスチス肺炎 ( 以下 PCP) に対する標準的な治療 ( および予防 ) 薬として承認されている 申請に至った経緯本邦では 本剤は未承認薬であるが 厚生労働省エイズ治療薬研究班 ( 班長東京医科大学主任教授福武勝幸 ) によりエイズ治療研究を目的に個人輸入され 国内医療機関に提供されている また 国立国際医療センターエイズ治療 研究開発センターも エイズ治療薬研究班とは別に個人輸入しており 本剤が PCP 治療の選択肢の一つとして使用されている この状況を踏まえ 日本エイズ学会よりニューモシスチス肺炎の治療および予防を目的とする本剤懸濁液の開発に関する要望書が提出され 厚生労働省医政局研究開発振興課および厚生労働省医薬食品局審査管理課より平成 21 年 9 月 28 日付医政研発 0928 第 1 号および薬食審査発 0928 第 1 号にて 医療上の必要性が高い未承認の医薬品 として開発の要望に関する意見に対する企業見解の提出が要請された グラクソ スミスクライン株式会社は この要請を受け 本剤懸濁液の本邦での承認申請の可能性について検討した その結果 本剤懸濁液は国内のガイドラインでは第三選択薬となっているものの 国内で承認されている ST 合剤 ( 第一選択薬 ) またはペンタミジン ( 第二選択薬 ) を使用した半数以上の患者で副作用により投与の継続を断念せざるを得ない状況にあり 本疾患の重篤性を考慮すると 本剤懸濁液は PCP の治療 一次予防 (CD4<200 /mm 3 の HIV 患者 ) および二次予防 ( 再発抑制 ) に必要不可欠な薬剤であると評価した また 海外で本剤の本適応疾患を対象とした臨床試験により有用性が確認され 評価に足る臨床成績は十分に揃っていること 本邦での臨床試験成績をまとめた論文はないものの 日本人に対する十分な使用実績があり 有用性を推測することは可能であることから 日本人患者を対象とした臨床試験を実施することなく 本邦で申請可能であるとの企業見解を平成 21 年 11 月 6 日付けで厚生労働省医薬食品局審査管理課宛に提出した この企業見解に基づき 厚生労働省医政局研究開発振興課および厚生労働省医薬食品局審査管理課より 平成 22 年 5 月 21 日付医政研発 0521 第 1 号にて正式な開発要請がなされたことから 本剤懸濁液の製造販売承認申請を行うこととした 申請資料の構成本申請資料は 次に示す資料構成で作成した 1 CMC: 海外承認申請資料 (NDA/MAA) を基に更新情報を反映させて作成した第 3 部から第 2 部を作成した 2 非臨床 : 海外承認申請資料 (NDA/MAA) を基に第 2 部を作成した Oct :22: p. 1
4 3 臨床 : 海外承認申請資料 (NDA/MAA) を基に第 2 部を作成した さらに 市販後安全 性情報として直近 3 年間の PSUR(2005 年 12 月 1 日 ~2008 年 11 月 30 日 ) および日本人 患者における使用経験を提示した 起原または発見の経緯および開発の経緯起原または発見の経緯および開発の経緯については 平成 13 年 6 月 21 日付医薬審発第 899 号厚生労働省医薬局審査管理課長通知 新医薬品の製造または輸入の承認申請に際し承認申請書に添付すべき資料の作成要領について の別紙 2 の 5(1) に作成要領が示されているが その中の 当該内容が第 2 部 (5) に記載できる場合は 第 1 部において提出を省略することができる との記述をもとに 当該内容をおもに第 2 部 (5) に記載した 表 に 第 2 部での当該内容の記載場所を示す また 開発の経緯図を図 に示す 表 第 1 部 (5) に関する内容の第 2 部における記載場所 第 1 部 (5) に記載する内容 第 2 部での記載場所 起原 薬理学的分類 病態 診断および治療 ニューモシスチス肺炎の臨床的 / 病態生理学的側面 現在の治療法およびその問題点 製剤の特徴 本剤の特徴 開発計画 開発の経緯 臨床開発計画 申請製剤 臨床試験に使用した製剤 2.3.P.2 製剤開発の経緯 非臨床試験成績 ( 薬理 薬物動態 毒性 ) 2.4 非臨床試験の概括評価 薬理試験 薬物動態試験 毒性試験 Oct :22: p. 2
5 図 開発の経緯図 p. 3 Oct :22:11
6 図 開発の経緯図 ( 続き ) p. 4 Oct :22:11
7 1.6. 外国における使用状況等に関する資料本項では 以下の資料を添付した 米国における添付文書の原文および和訳 英国における添付文書の原文および和訳 企業中核データシート (COMPANY CORE DATASHEET) の原文 本剤は 初めて米国で ニューモシスチス肺炎 ( 以下 PCP) に対する治療薬としてアトバコン 250mg 錠 (Mepron 250mg Tablet) が承認 (1992 年 11 月 25 日 ) され 同 250mg 錠 (Wellvone 250mg Tablet) が ドイツ (1994 年 8 月 4 日 ) 英国(1994 年 8 月 23 日 ) およびフランス (1994 年 9 月 15 日 ) でそれぞれ承認された その後 アトバコンの吸収を高めるためにアトバコンの超微粒子を用いた懸濁液剤が開発され 当該懸濁液剤 (Mepron / Wellvone Oral Suspension:750mg/5mL) の承認を米国 (1995 年 2 月 8 日 ) フランス(1996 年 8 月 21 日 ) 英国(1997 年 3 月 25 日 ) およびドイツ (1997 年 4 月 17 日 ) でそれぞれ取得した さらに 同懸濁液剤に対する追加効能 (PCP 予防 ) が米国において承認 (1999 年 1 月 5 日 ) されている アトバコン 250mg 錠 (Mepron/Wellvone 250mg Tablet) または懸濁液剤 (Mepron/Wellvone Oral Suspension:750mg/5mL) が 米国 カナダ フランス 英国 ドイツを含む海外 21 ヵ国 (2011 年 2 月現在 ) で PCP に対する治療 ( 予防 ) 薬として承認されている 主要国 ( 米国および英国 ) における懸濁液剤の承認状況を表 に示す 表 国 / 地域名 販売名 承認年月日 米国 Mepron ( 治療 ) 1995 年 2 月 8 日 ( 予防 ) 1999 年 1 月 5 日 英国 Wellvone ( 治療 ) 1997 年 3 月 25 日 米国および英国における懸濁液剤の承認の状況 剤型 含量 経口懸濁液剤 750 mg / 5mL 経口懸濁液剤 750 mg / 5mL 効能 効果用法 用量 効能 効果 : トリメトプリム/ スルファメトキサゾール (TMP-SMX) 合剤に不耐容の患者におけるニューモシスチス カリニ肺炎 (PCP) の予防 その他 TMP-SMX 合剤に不耐容の患者における軽度から中等度の PCP の急性期経口治療用法 用量 : PCP の予防 : 成人および青年 (13~16 歳 ):1,500 mg(10 ml) を 1 日 1 回食後に経口投与 軽度から中等度の PCP の治療 : 成人および青年 (13~16 歳 ): 750 mg(5 ml) を 1 日 2 回 21 日間 食後に経口投与 (1 日総投与量 1,500 mg) 効能 効果 : コトリモキサゾールによる治療に不耐容の患者における軽度から中等度のニューモシスチス肺炎 (PCP)( 室内気吸入下での肺胞気 動脈血酸素分圧較差 [(A-a)DO 2] が 45 mmhg(6 kpa) 以下かつ動脈血酸素分圧 (PaO 2) が 60 mmhg(8 kpa) 以上 ) の急性期治療用量 用法 ( 成人 ): ニューモシスチス肺炎: 750 mg を 1 日 2 回 (5 ml を朝と晩に 1 回ずつ )21 日間 食後に経口投与 p. 1
8 MEPRON (atovaquone) Suspension PRESCRIBING INFORMATION DESCRIPTION MEPRON (atovaquone) is an antiprotozoal agent. The chemical name of atovaquone is trans- 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione. Atovaquone is a yellow crystalline solid that is practically insoluble in water. It has a molecular weight of and the molecular formula C 22 H 19 ClO 3. The compound has the following structural formula: MEPRON Suspension is a formulation of micro-fine particles of atovaquone. The atovaquone particles, reduced in size to facilitate absorption, are significantly smaller than those in the previously marketed tablet formulation. MEPRON Suspension is for oral administration and is bright yellow with a citrus flavor. Each teaspoonful (5 ml) contains 750 mg of atovaquone and the inactive ingredients benzyl alcohol, flavor, poloxamer 188, purified water, saccharin sodium, and xanthan gum. MICROBIOLOGY Mechanism of Action: Atovaquone is a hydroxy-1,4-naphthoquinone, an analog of ubiquinone, with antipneumocystis activity. The mechanism of action against Pneumocystis carinii has not been fully elucidated. In Plasmodium species, the site of action appears to be the cytochrome bc 1 complex (Complex III). Several metabolic enzymes are linked to the mitochondrial electron transport chain via ubiquinone. Inhibition of electron transport by atovaquone will result in indirect inhibition of these enzymes. The ultimate metabolic effects of such blockade may include inhibition of nucleic acid and ATP synthesis. Activity In Vitro: Several laboratories, using different in vitro methodologies, have shown the IC 50 (50% inhibitory concentration) of atovaquone against rat P. carinii to be in the range of 0.1 to 3.0 mcg/ml. Drug Resistance: Phenotypic resistance to atovaquone in vitro has not been demonstrated for P. carinii. However, in 2 patients who developed P. carinii pneumonia (PCP) after prophylaxis with atovaquone, DNA sequence analysis identified mutations in the predicted amino acid sequence of P. carinii cytochrome b (a likely target site for atovaquone). The clinical significance of this is unknown. 1
9 CLINICAL PHARMACOLOGY Pharmacokinetics: Absorption: Atovaquone is a highly lipophilic compound with low aqueous solubility. The bioavailability of atovaquone is highly dependent on formulation and diet. The suspension formulation provides an approximately 2-fold increase in atovaquone bioavailability in the fasting or fed state compared to the previously marketed tablet formulation. The absolute bioavailability of a 750-mg dose of MEPRON Suspension administered under fed conditions in 9 HIV-infected (CD4 >100 cells/mm 3 ) volunteers was 47% ± 15%. In the same study, the bioavailability of a 750-mg dose of the previously marketed tablet formulation was 23% ± 11%. Administering atovaquone with food enhances its absorption by approximately 2 fold. In one study, 16 healthy volunteers received a single dose of 750 mg MEPRON Suspension after an overnight fast and following a standard breakfast (23 g fat: 610 kcal). The mean (±SD) area under the concentration-time curve (AUC) values were 324 ± 115 and 801 ± 320 hr mcg/ml under fasting and fed conditions, respectively, representing a 2.6 ± 1.0-fold increase. The effect of food (23 g fat: 400 kcal) on plasma atovaquone concentrations was also evaluated in a multiple-dose, randomized, crossover study in 19 HIV-infected volunteers (CD4 <200 cells/mm 3 ) receiving daily doses of 500 mg MEPRON Suspension. AUC was 280 ± 114 hr mcg/ml when atovaquone was administered with food as compared to 169 ± 77 hr mcg/ml under fasting conditions. Maximum plasma atovaquone concentration (C max ) was 15.1 ± 6.1 and 8.8 ± 3.7 mcg/ml when atovaquone was administered with food and under fasting conditions, respectively. Dose Proportionality: Plasma atovaquone concentrations do not increase proportionally with dose. When MEPRON Suspension was administered with food at dosage regimens of 500 mg once daily, 750 mg once daily, and 1,000 mg once daily, average steady-state plasma atovaquone concentrations were 11.7 ± 4.8, 12.5 ± 5.8, and 13.5 ± 5.1 mcg/ml, respectively. The corresponding C max concentrations were 15.1 ± 6.1, 15.3 ± 7.6, and 16.8 ± 6.4 mcg/ml. When MEPRON Suspension was administered to 5 HIV-infected volunteers at a dose of 750 mg twice daily, the average steady-state plasma atovaquone concentration was 21.0 ± 4.9 mcg/ml and C max was 24.0 ± 5.7 mcg/ml. The minimum plasma atovaquone concentration (C min ) associated with the 750-mg twice-daily regimen was 16.7 ± 4.6 mcg/ml. Distribution: Following the intravenous administration of atovaquone, the volume of distribution at steady state (Vd ss ) was 0.60 ± 0.17 L/kg (n = 9). Atovaquone is extensively bound to plasma proteins (99.9%) over the concentration range of 1 to 90 mcg/ml. In 3 HIV-infected children who received 750 mg atovaquone as the tablet formulation 4 times daily for 2 weeks, the cerebrospinal fluid concentrations of atovaquone were 0.04, 0.14, and 0.26 mcg/ml, representing less than 1% of the plasma concentration. Elimination: The plasma clearance of atovaquone following intravenous (IV) administration in 9 HIV-infected volunteers was 10.4 ± 5.5 ml/min (0.15 ± 0.09 ml/min/kg). The half-life of atovaquone was 62.5 ± 35.3 hours after IV administration and ranged from 67.0 ± 33.4 to 77.6 ± 23.1 hours across studies following administration of MEPRON Suspension. The half-life of atovaquone is long due to presumed enterohepatic cycling and eventual fecal elimination. In a study where 14 C-labelled atovaquone was administered to healthy volunteers, greater than 94% of the dose was recovered as unchanged atovaquone in the feces over 21 days. There was little or no excretion of atovaquone in the urine (less than 0.6%). There is indirect evidence that 2
10 atovaquone may undergo limited metabolism; however, a specific metabolite has not been identified. Special Populations: Pediatrics: In a study of MEPRON Suspension in 27 HIV-infected, asymptomatic infants and children between 1 month and 13 years of age, the pharmacokinetics of atovaquone were age dependent. These patients were dosed once daily with food for 12 days. The average steady-state plasma atovaquone concentrations in the 24 patients with available concentration data are shown in Table 1. Table 1. Average Steady-State Plasma Atovaquone Concentrations in Pediatric Patients Dose of MEPRON Suspension 10 mg/kg 30 mg/kg 45 mg/kg Age 1-3 months 5.9 (n = 1) >3-24 months 5.7 ± 5.1 (n = 4) >2-13 years 16.8 ± 6.4 (n = 4) Average C ss in mcg/ml (mean ± SD) 27.8 ± 5.8 (n = 4) 9.8 ± 3.2 (n = 4) 37.1 ± 10.9 (n = 3) 15.4 ± 6.6 (n = 4) Hepatic/Renal Impairment: The pharmacokinetics of atovaquone have not been studied in patients with hepatic or renal impairment. Drug Interactions: Rifampin: In a study with 13 HIV-infected volunteers, the oral administration of rifampin 600 mg every 24 hours with MEPRON Suspension 750 mg every 12 hours resulted in a 52% ± 13% decrease in the average steady-state plasma atovaquone concentration and a 37% ± 42% increase in the average steady-state plasma rifampin concentration. The half-life of atovaquone decreased from 82 ± 36 hours when administered without rifampin to 50 ± 16 hours with rifampin. Rifabutin, another rifamycin, is structurally similar to rifampin and may possibly have some of the same drug interactions as rifampin. No interaction trials have been conducted with MEPRON and rifabutin. Trimethoprim/Sulfamethoxazole (TMP-SMX): The possible interaction between atovaquone and TMP-SMX was evaluated in 6 HIV-infected adult volunteers as part of a larger multiple-dose, dose-escalation, and chronic dosing study of MEPRON Suspension. In this crossover study, MEPRON Suspension 500 mg once daily, or TMP-SMX tablets (160 mg trimethoprim and 800 mg sulfamethoxazole) twice daily, or the combination were administered with food to achieve steady state. No difference was observed in the average steady-state plasma atovaquone concentration after coadministration with TMP-SMX. Coadministration of MEPRON with TMP-SMX resulted in a 17% and 8% decrease in average steady-state concentrations of trimethoprim and sulfamethoxazole in plasma, respectively. This effect is minor and would not be expected to produce clinically significant events. Zidovudine: Data from 14 HIV-infected volunteers who were given atovaquone tablets 750 mg every 12 hours with zidovudine 200 mg every 8 hours showed a 24% ± 12% decrease in zidovudine apparent oral clearance, leading to a 35% ± 23% increase in plasma zidovudine AUC. 3
11 The glucuronide metabolite:parent ratio decreased from a mean of 4.5 when zidovudine was administered alone to 3.1 when zidovudine was administered with atovaquone tablets. This effect is minor and would not be expected to produce clinically significant events. Zidovudine had no effect on atovaquone pharmacokinetics. Relationship Between Plasma Atovaquone Concentration and Clinical Outcome: In a comparative study of atovaquone tablets with TMP-SMX for oral treatment of mild-to-moderate Pneumocystis carinii pneumonia (PCP) (see INDICATIONS AND USAGE), where AIDS patients received 750 mg atovaquone tablets 3 times daily for 21 days, the mean steady-state atovaquone concentration was 13.9 ± 6.9 mcg/ml (n = 133). Analysis of these data established a relationship between plasma atovaquone concentration and successful treatment. This is shown in Table 2. Table 2. Relationship Between Plasma Atovaquone Concentration and Successful Treatment Steady-State Plasma Atovaquone Concentrations Successful Treatment* (No. Successes/No. in Group) (%) (mcg/ml) Observed Predicted 0 to <5 0/6 (0%) 1.5/6 (25%) 5 to <10 18/26 (69%) 14.7/26 (57%) 10 to <15 30/38 (79%) 31.9/38 (84%) 15 to <20 18/19 (95%) 18.1/19 (95%) 20 to <25 18/18 (100%) 17.8/18 (99%) 25+ 6/6 (100%) 6/6 (100%) * Successful treatment was defined as improvement in clinical and respiratory measures persisting at least 4 weeks after cessation of therapy. This was based on data from patients for which both outcome and steady-state plasma atovaquone concentration data are available. Based on logistic regression analysis. A dosing regimen of MEPRON Suspension for the treatment of mild-to-moderate PCP has been selected to achieve average plasma atovaquone concentrations of approximately 20 mcg/ml, because this plasma concentration was previously shown to be well tolerated and associated with the highest treatment success rates (Table 2). In an open-label PCP treatment study with MEPRON Suspension, dosing regimens of 1,000 mg once daily, 750 mg twice daily, 1,500 mg once daily, and 1,000 mg twice daily were explored. The average steady-state plasma atovaquone concentration achieved at the 750-mg twice-daily dose given with meals was 22.0 ± 10.1 mcg/ml (n = 18). INDICATIONS AND USAGE MEPRON Suspension is indicated for the prevention of Pneumocystis carinii pneumonia in patients who are intolerant to trimethoprim-sulfamethoxazole (TMP-SMX). MEPRON Suspension is also indicated for the acute oral treatment of mild-to-moderate PCP in patients who are intolerant to TMP-SMX. Prevention of PCP: The indication for prevention of PCP is based on the results of 2 clinical trials comparing MEPRON Suspension to dapsone or aerosolized pentamidine in HIV-infected 4
12 adult and adolescent patients at risk of PCP (CD4 count <200 cells/mm 3 or a prior episode of PCP) and intolerant to TMP-SMX. Dapsone Comparative Study: This randomized, open-label trial enrolled a total of 1,057 patients at 48 study centers. Patients were randomized to receive 1,500 mg MEPRON Suspension once daily (n = 536) or 100 mg dapsone once daily (n = 521). Median follow-up was 24 months. Patients randomized to the dapsone arm who were seropositive for Toxoplasma gondii and had a CD4 count <100 cells/mm 3 also received pyrimethamine and folinic acid. PCP event rates are shown in Table 3. There was no significant difference in mortality rates between the groups. Aerosolized Pentamidine Comparative Study: This randomized, open-label trial enrolled a total of 549 patients at 35 study centers. Patients were randomized to receive 1,500 mg MEPRON Suspension once daily (n = 175), 750 mg MEPRON Suspension once daily (n = 188), or 300 mg aerosolized pentamidine once monthly (n = 186). Median follow-up was 11.3 months. The results of the PCP event rates appear in Table 3. There were no significant differences in mortality rates among the groups. Table 3. Confirmed or Presumed/Probable PCP Events (As-Treated Analysis)* Study Study Aerosolized Pentamidine 300 mg/month (n = 169) Assessment Atovaquone 1,500 mg/day (n = 527) Dapsone 100 mg/day (n = 510) Atovaquone 750 mg/day (n = 188) Atovaquone 1,500 mg/day (n = 172) % 15% 19% 23% 18% 17% Relative Risk (CI) (0.57, 1.04) (0.86, 2.50) (0.63, 2.06) * Those events occurring during or within 30 days of stopping assigned treatment. Relative risk <1 favors atovaquone and values >1 favor comparator. These trials were designed to show superiority of atovaquone to the comparator. This was not shown. The confidence level of the interval for the dapsone comparative study was 95% and for the pentamidine comparative study was 97.5%. An analysis of all PCP events (intent-to-treat analysis) showed results similar to those above. Treatment of PCP: The indication for treatment of mild-to-moderate PCP is based on the results of comparative pharmacokinetic studies of the suspension and tablet formulations (see CLINICAL PHARMACOLOGY) and clinical efficacy studies of the tablet formulation which established a relationship between plasma atovaquone concentration and successful treatment. The results of a randomized, double-blind trial comparing MEPRON to TMP-SMX in AIDS patients with mild-to-moderate PCP (defined in the study protocol as an alveolar-arterial oxygen diffusion gradient [(A-a)DO 2 ] 1 45 mm Hg and PaO 2 60 mm Hg on room air) and a randomized trial comparing MEPRON to IV pentamidine isethionate in patients with mild-to-moderate PCP intolerant to trimethoprim or sulfa-antimicrobials are summarized below: TMP-SMX Comparative Study: This double-blind, randomized trial initiated in 1990 was designed to compare the safety and efficacy of MEPRON to that of TMP-SMX for the treatment 5
13 of AIDS patients with histologically confirmed PCP. Only patients with mild-to-moderate PCP were eligible for enrollment. A total of 408 patients were enrolled into the trial at 37 study centers. Eighty-six patients without histologic confirmation of PCP were excluded from the efficacy analyses. Of the 322 patients with histologically confirmed PCP, 160 were randomized to receive MEPRON and 162 to TMP-SMX. Study participants randomized to treatment with MEPRON were to receive 750 mg MEPRON (three 250-mg tablets) 3 times daily for 21 days and those randomized to TMP-SMX were to receive 320 mg TMP plus 1,600 mg SMX 3 times daily for 21 days. Therapy success was defined as improvement in clinical and respiratory measures persisting at least 4 weeks after cessation of therapy. Therapy failures included lack of response, treatment discontinuation due to an adverse experience, and unevaluable. There was a significant difference (P = 0.03) in mortality rates between the treatment groups. Among the 322 patients with confirmed PCP, 13 of 160 (8%) patients treated with MEPRON and 4 of 162 (2.5%) patients receiving TMP-SMX died during the 21-day treatment course or 8-week follow-up period. In the intent-to-treat analysis for all 408 randomized patients, there were 16 (8%) deaths in the arm treated with MEPRON and 7 (3.4%) deaths in the TMP-SMX arm (P = 0.051). Of the 13 patients treated with MEPRON who died, 4 died of PCP and 5 died with a combination of bacterial infections and PCP; bacterial infections did not appear to be a factor in any of the 4 deaths among TMP-SMX-treated patients. A correlation between plasma atovaquone concentrations and death was demonstrated; in general, patients with lower plasma concentrations were more likely to die. For those patients for whom day 4 plasma atovaquone concentration data are available, 5 (63%) of the 8 patients with concentrations <5 mcg/ml died during participation in the study. However, only 1 (2.0%) of the 49 patients with day 4 plasma atovaquone concentrations 5 mcg/ml died. Sixty-two percent of patients on MEPRON and 64% of patients on TMP-SMX were classified as protocol-defined therapy successes (Table 4). Table 4. Outcome of Treatment for PCP-Positive Patients Enrolled in the TMP-SMX Comparative Study Number of Patients (% of Total) Outcome of Therapy* MEPRON (n = 160) TMP-SMX (n = 162) P Value Therapy success 99 (62%) 103 (64%) 0.75 Therapy failure -Lack of response 28 (17%) 10 (6%) <0.01 -Adverse experience 11 (7%) 33 (20%) <0.01 -Unevaluable 22 (14%) 16 (10%) 0.28 Required alternate PCP therapy during study 55 (34%) 55 (34%) 0.95 * As defined by the protocol and described in study description above. 6
14 The failure rate due to lack of response was significantly larger for patients receiving MEPRON while the failure rate due to adverse experiences was significantly larger for patients receiving TMP-SMX. There were no significant differences in the effect of either treatment on additional indicators of response (i.e., arterial blood gas measurements, vital signs, serum LDH levels, clinical symptoms, and chest radiographs). Pentamidine Comparative Study: This unblinded, randomized trial initiated in 1991 was designed to compare the safety and efficacy of MEPRON to that of pentamidine for the treatment of histologically confirmed mild or moderate PCP in AIDS patients. Approximately 80% of the patients either had a history of intolerance to trimethoprim or sulfa-antimicrobials (the primary therapy group) or were experiencing intolerance to TMP-SMX with treatment of an episode of PCP at the time of enrollment in the study (the salvage treatment group). Patients randomized to MEPRON were to receive 750 mg atovaquone (three 250-mg tablets) 3 times daily for 21 days and those randomized to pentamidine isethionate were to receive a 3- to 4-mg/kg single IV infusion daily for 21 days. A total of 174 patients were enrolled into the trial at 22 study centers. Thirty-nine patients without histologic confirmation of PCP were excluded from the efficacy analyses. Of the 135 patients with histologically confirmed PCP, 70 were randomized to receive MEPRON and 65 to pentamidine. One hundred and ten (110) of these were in the primary therapy group and 25 were in the salvage therapy group. One patient in the primary therapy group randomized to receive pentamidine did not receive study medication. There was no difference in mortality rates between the treatment groups. Among the 135 patients with confirmed PCP, 10 of 70 (14%) patients randomized to MEPRON and 9 of 65 (14%) patients randomized to pentamidine died during the 21-day treatment course or 8-week follow-up period. In the intent-to-treat analysis for all randomized patients, there were 11 (12.5%) deaths in the arm treated with MEPRON and 12 (14%) deaths in the pentamidine arm. For those patients for whom day 4 plasma atovaquone concentrations are available, 3 of 5 (60%) patients with concentrations <5 mcg/ml died during participation in the study. However, only 2 of 21 (9%) patients with day 4 plasma concentrations 5 mcg/ml died. The therapeutic outcomes for the 134 patients who received study medication in this trial are presented in Table 5. 7
15 Table 5. Outcome of Treatment for PCP-Positive Patients Enrolled in the Pentamidine Comparative Study Outcome of Therapy Primary Treatment MEPRON (n = 56) Pentamidine (n = 53) P Value Salvage Treatment MEPRON (n = 14) Pentamidine (n = 11) P Value Therapy success 32 (57%) 21 (40%) (93%) 7 (64%) 0.14 Therapy failure -Lack of response 16 (29%) 9 (17%) Adverse experience 2 (3.6%) 19 (36%) < (27%) Unevaluable 6 (11%) 4 (8%) (7%) 1 (9%) 1.00 Required alternate PCP therapy during study 19 (34%) 29 (55%) (36%) 0.03 CONTRAINDICATIONS MEPRON Suspension is contraindicated for patients who develop or have a history of potentially life-threatening allergic reactions to any of the components of the formulation. WARNINGS Clinical experience with MEPRON for the treatment of PCP has been limited to patients with mild-to-moderate PCP [(A-a)DO 2 45 mm Hg]. Treatment of more severe episodes of PCP has not been systematically studied with this agent. Also, the efficacy of MEPRON in patients who are failing therapy with TMP-SMX has not been systematically studied. PRECAUTIONS General: Absorption of orally administered MEPRON is limited but can be significantly increased when the drug is taken with food. Plasma atovaquone concentrations have been shown to correlate with the likelihood of successful treatment and survival. Therefore, parenteral therapy with other agents should be considered for patients who have difficulty taking MEPRON with food (see CLINICAL PHARMACOLOGY). Gastrointestinal disorders may limit absorption of orally administered drugs. Patients with these disorders also may not achieve plasma concentrations of atovaquone associated with response to therapy in controlled trials. Based upon the spectrum of in vitro antimicrobial activity, atovaquone is not effective therapy for concurrent pulmonary conditions such as bacterial, viral, or fungal pneumonia or mycobacterial diseases. Clinical deterioration in patients may be due to infections with other pathogens, as well as progressive PCP. All patients with acute PCP should be carefully evaluated for other possible causes of pulmonary disease and treated with additional agents as appropriate. Rare cases of hepatitis, elevated liver function tests, and one case of fatal liver failure have been reported in patients treated with atovaquone. A causal relationship between atovaquone use and these events could not be established because of numerous confounding medical conditions and concomitant drug therapies. (See ADVERSE REACTIONS.) If it is necessary to treat patients with severe hepatic impairment, caution is advised and administration should be closely monitored. 8
16 Information for Patients: The importance of taking the prescribed dose of MEPRON should be stressed. Patients should be instructed to take their daily doses of MEPRON with meals, as the presence of food will significantly improve the absorption of the drug. Drug Interactions: Atovaquone is highly bound to plasma protein (>99.9%). Therefore, caution should be used when administering MEPRON concurrently with other highly plasma protein-bound drugs with narrow therapeutic indices, as competition for binding sites may occur. The extent of plasma protein binding of atovaquone in human plasma is not affected by the presence of therapeutic concentrations of phenytoin (15 mcg/ml), nor is the binding of phenytoin affected by the presence of atovaquone. Rifampin: Coadministration of rifampin and MEPRON Suspension results in a significant decrease in average steady-state plasma atovaquone concentrations (see CLINICAL PHARMACOLOGY: Drug Interactions). Alternatives to rifampin should be considered during the course of PCP treatment with MEPRON. Rifabutin, another rifamycin, is structurally similar to rifampin and may possibly have some of the same drug interactions as rifampin. No interaction trials have been conducted with MEPRON and rifabutin. Drug/Laboratory Test Interactions: It is not known if MEPRON interferes with clinical laboratory test or assay results. Carcinogenesis, Mutagenesis, Impairment of Fertility: Carcinogenicity studies in rats were negative; 24-month studies in mice showed treatment-related increases in incidence of hepatocellular adenoma and hepatocellular carcinoma at all doses tested which ranged from 1.4 to 3.6 times the average steady-state plasma concentrations in humans during acute treatment of Pneumocystis carinii pneumonia. Atovaquone was negative with or without metabolic activation in the Ames Salmonella mutagenicity assay, the Mouse Lymphoma mutagenesis assay, and the Cultured Human Lymphocyte cytogenetic assay. No evidence of genotoxicity was observed in the in vivo Mouse Micronucleus assay. Pregnancy: Pregnancy Category C. Atovaquone was not teratogenic and did not cause reproductive toxicity in rats at plasma concentrations up to 2 to 3 times the estimated human exposure. Atovaquone caused maternal toxicity in rabbits at plasma concentrations that were approximately one half the estimated human exposure. Mean fetal body lengths and weights were decreased and there were higher numbers of early resorption and post-implantation loss per dam. It is not clear whether these effects were caused by atovaquone directly or were secondary to maternal toxicity. Concentrations of atovaquone in rabbit fetuses averaged 30% of the concurrent maternal plasma concentrations. In a separate study in rats given a single 14 C-radiolabelled dose, concentrations of radiocarbon in rat fetuses were 18% (middle gestation) and 60% (late gestation) of concurrent maternal plasma concentrations. There are no adequate and well-controlled studies in pregnant women. MEPRON should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Nursing Mothers: It is not known whether atovaquone is excreted into human milk. Because many drugs are excreted into human milk, caution should be exercised when MEPRON is administered to a nursing woman. In a rat study, atovaquone concentrations in the milk were 30% of the concurrent atovaquone concentrations in the maternal plasma. Pediatric Use: Evidence of safety and effectiveness in pediatric patients has not been established. A relationship between plasma atovaquone concentrations and successful treatment of PCP has been established in adults (see Table 2). In a study of MEPRON Suspension in 27 HIV-infected, asymptomatic infants and children between 1 month and 13 years of age, the 9
17 pharmacokinetics of atovaquone were age-dependent (see CLINICAL PHARMACOLOGY: Special Populations). No drug-related treatment-limiting adverse events were observed in the pharmacokinetic study. Geriatric Use: Clinical studies of MEPRON did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. ADVERSE REACTIONS Because many patients who participated in clinical trials with MEPRON had complications of advanced HIV disease, it was often difficult to distinguish adverse events caused by MEPRON from those caused by underlying medical conditions. There were no life-threatening or fatal adverse experiences caused by MEPRON. PCP Prevention Studies: In the dapsone comparative study of MEPRON Suspension, adverse experience data were collected only for treatment-limiting events. Among the entire population (n = 1,057), treatment-limiting events occurred at similar frequencies in patients treated with MEPRON Suspension or dapsone (Table 6). Among patients who were taking neither dapsone nor atovaquone at enrollment (n = 487), treatment-limiting events occurred in 43% of patients treated with dapsone and 20% of patients treated with MEPRON Suspension (P <0.001). In both populations, the type of treatment-limiting events differed between the 2 treatment arms. Hypersensitivity reactions (rash, fever, allergic reaction) and anemia were more common in patients treated with dapsone, while gastrointestinal events (nausea, diarrhea, and vomiting) were more common in patients treated with MEPRON Suspension. Table 6. Treatment-Limiting Adverse Experiences in the Dapsone Comparative PCP Prevention Study Percentage of Patients with Treatment-Limiting Adverse Experience Patients Not Taking Either Drug at MEPRON 1,500 mg/day (n = 536) All Patients Dapsone 100 mg/day (n = 521) MEPRON 1,500 mg/day (n = 238) Enrollment Dapsone 100 mg/day (n = 249) Treatment-Limiting Adverse Experience Any event 24.4% 25.9% 20.2% 43.4% Rash 6.3% 8.8% 7.6% 16.1% Nausea 4.1% 0.6% 2.5% 0.8% Diarrhea 3.2% 0.2% 2.1% 0.4% Vomiting 2.2% 0.6% 1.3% 0.8% Allergic reaction 1.1% 2.9% 0.8% 4.8% Fever 0.6% 2.9% 0% 5.6% Anemia 0% 1.5% 0% 2.0% 10
18 Table 7 summarizes the clinical adverse experiences reported by 20% of patients in any group in the aerosolized pentamidine comparative study of MEPRON Suspension (n = 549), regardless of attribution. The incidence of adverse experiences at the recommended dose was similar to that seen with aerosolized pentamidine. Rash was the only individual adverse experience that occurred significantly more commonly in patients treated with both dosages of MEPRON Suspension (39% to 46%) than in patients treated with aerosolized pentamidine (28%). Among patients treated with MEPRON Suspension, there was no evidence of a dose-related increase in the incidence of adverse experiences. Treatment-limiting adverse experiences occurred less often in patients treated with aerosolized pentamidine (7%) than in patients treated with 1,500 mg MEPRON Suspension once daily (25%, P 0.001) or 750 mg MEPRON Suspension once daily (16%, P = 0.004). The most common adverse experiences requiring discontinuation of dosing in the group receiving 1,500 mg MEPRON Suspension once daily were rash (6%), diarrhea (4%), and nausea (3%). The most common adverse experience requiring discontinuation of dosing in the group receiving aerosolized pentamidine was bronchospasm (2%). Table 7. Treatment-Emergent Adverse Experiences in the Aerosolized Pentamidine Comparative PCP Prevention Study Percentage of Patients with Treatment-Emergent Adverse Experience MEPRON 1,500 mg/day (n = 175) MEPRON 750 mg/day (n = 188) Aerosolized Pentamidine (n = 186) Treatment-Emergent Adverse Experience Diarrhea 42% 42% 35% Rash 39% 46% 28% Headache 28% 31% 22% Nausea 26% 32% 23% Cough increased 25% 25% 31% Fever 25% 31% 18% Rhinitis 24% 18% 17% Asthenia 22% 31% 31% Infection 22% 18% 19% Abdominal pain 20% 21% 20% Dyspnea 15% 21% 16% Vomiting 15% 22% 11% Patients discontinuing therapy due to an adverse experience Patients reporting at least 1 adverse experience 25% 16% 7% 98% 96% 89% Other events occurring in 10% of the patients receiving the recommended dose of MEPRON included sweating, flu syndrome, pain, sinusitis, pruritus, insomnia, depression, and myalgia. Bronchospasm occurred more frequently in patients receiving aerosolized pentamidine (11%) than in patients receiving MEPRON 1,500 mg/day (4%) and MEPRON 750 mg/day (2%). 11
19 Neither MEPRON nor aerosolized pentamidine was associated with a substantial change from baseline values in any measured laboratory parameter, nor were there any significant differences in any measured laboratory parameter between MEPRON and aerosolized pentamidine. Some patients had laboratory abnormalities considered serious by the investigator or that contributed to discontinuation of therapy. PCP Treatment Studies: Table 8 summarizes all the clinical adverse experiences reported by 5% of the study population during the TMP-SMX comparative study of MEPRON (n = 408), regardless of attribution. The incidence of adverse experiences with MEPRON Suspension at the recommended dose was similar to that seen with the tablet formulation of atovaquone. Table 8. Treatment-Emergent Adverse Experiences in the TMP-SMX Comparative PCP Treatment Study Percentage of Patients with Treatment-Emergent Adverse Experience Treatment-Emergent Adverse Experience MEPRON (n = 203) Rash (including maculopapular) 23% 34% Nausea 21% 44% Diarrhea 19% 7% Headache 16% 22% Vomiting 14% 35% Fever 14% 25% Insomnia 10% 9% Asthenia 8% 8% Pruritus 5% 9% Monilia, oral 5% 10% Abdominal pain 4% 7% Constipation 3% 17% Dizziness 3% 8% Patients discontinuing therapy due to an adverse experience Patients reporting at least 1 adverse experience TMP-SMX (n = 205) 9% 24% 63% 65% Although an equal percentage of patients receiving MEPRON and TMP-SMX reported at least 1 adverse experience, more patients receiving TMP-SMX required discontinuation of therapy due to an adverse event. Twenty-four percent of patients receiving TMP-SMX were prematurely discontinued from therapy due to an adverse experience versus 9% of patients receiving MEPRON. Four percent of patients receiving MEPRON had therapy discontinued due to development of rash. The majority of cases of rash among patients receiving MEPRON were mild and did not require the discontinuation of dosing. The only other clinical adverse experience that led to premature discontinuation of dosing of MEPRON by more than 1 patient was vomiting (<1%). The most common adverse experience requiring discontinuation of dosing in the TMP-SMX group was rash (8%). 12
20 Laboratory test abnormalities reported for 5% of the study population during the treatment period are summarized in Table 9. Two percent of patients treated with MEPRON and 7% of patients treated with TMP-SMX had therapy prematurely discontinued due to elevations in ALT/AST. In general, patients treated with MEPRON developed fewer abnormalities in measures of hepatocellular function (ALT, AST, alkaline phosphatase) or amylase values than patients treated with TMP-SMX. Table 9. Treatment-Emergent Laboratory Test Abnormalities in the TMP-SMX Comparative PCP Treatment Study Percentage of Patients Developing a Laboratory Test Abnormality Laboratory Test Abnormality MEPRON TMP-SMX Anemia (Hgb<8.0 g/dl) 6% 7% Neutropenia (ANC<750 cells/mm 3 ) 3% 9% Elevated ALT (>5 x ULN) 6% 16% Elevated AST (>5 x ULN) 4% 14% Elevated alkaline phosphatase (>2.5 x ULN) 8% 6% Elevated amylase (>1.5 x ULN) 7% 12% Hyponatremia (<0.96 x LLN) 7% 26% ULN = upper limit of normal range. LLN = lower limit of normal range. Table 10 summarizes the clinical adverse experiences reported by 5% of the primary therapy study population (n = 144) during the comparative trial of MEPRON and intravenous pentamidine, regardless of attribution. A slightly lower percentage of patients who received MEPRON reported occurrence of adverse events than did those who received pentamidine (63% vs 72%). However, only 7% of patients discontinued treatment with MEPRON due to adverse events, while 41% of patients who received pentamidine discontinued treatment for this reason (P<0.001). Of the 5 patients who discontinued therapy with MEPRON, 3 reported rash (4%). Rash was not severe in any patient. No other reason for discontinuation of MEPRON was cited more than once. The most frequently cited reasons for discontinuation of pentamidine therapy were hypoglycemia (11%) and vomiting (9%). 13
21 Table 10. Treatment-Emergent Adverse Experiences in the Pentamidine Comparative PCP Treatment Study (Primary Therapy Group) Percentage of Patients with Treatment-Emergent Adverse Experience Treatment-Emergent Adverse Experience MEPRON (n = 73) Fever 40% 25% Nausea 22% 37% Rash 22% 13% Diarrhea 21% 31% Insomnia 19% 14% Headache 18% 28% Vomiting 14% 17% Cough 14% 1% Abdominal pain 10% 11% Pain 10% 10% Sweat 10% 3% Monilia, oral 10% 3% Asthenia 8% 14% Dizziness 8% 14% Anxiety 7% 10% Anorexia 7% 10% Sinusitis 7% 6% Dyspepsia 5% 10% Rhinitis 5% 7% Taste perversion 3% 13% Hypoglycemia 1% 15% Hypotension 1% 10% Patients discontinuing therapy due to an adverse experience Patients reporting at least 1 adverse experience Pentamidine (n = 71) 7% 41% 63% 72% Laboratory test abnormalities reported in 5% of patients in the pentamidine comparative study are presented in Table 11. Laboratory abnormality was reported as the reason for discontinuation of treatment in 2 of 73 patients who received MEPRON. One patient (1%) had elevated creatinine and BUN levels and 1 patient (1%) had elevated amylase levels. Laboratory abnormalities were the sole or contributing factor in 14 patients who prematurely discontinued pentamidine therapy. In the 71 patients who received pentamidine, laboratory parameters most frequently reported as reasons for discontinuation were hypoglycemia (11%), elevated creatinine levels (6%), and leukopenia (4%). 14
22 Table 11. Treatment-Emergent Laboratory Test Abnormalities in the Pentamidine Comparative PCP Treatment Study Percentage of Patients Developing a Laboratory Test Laboratory Test Abnormality Abnormality MEPRON Pentamidine Anemia (Hgb<8.0 g/dl) 4% 9% Neutropenia (ANC<750 cells/mm 3 ) 5% 9% Hyponatremia (<0.96 x LLN) 10% 10% Hyperkalemia (>1.18 x ULN) 0% 5% Alkaline phosphatase (>2.5 x ULN) 5% 2% Hyperglycemia (>1.8 x ULN) 9% 13% Elevated AST (>5 x ULN) 0% 5% Elevated amylase (>1.5 x ULN) 8% 4% Elevated creatinine (>1.5 x ULN) 0% 7% ULN = upper limit of normal range. LLN = lower limit of normal range. Postmarketing Experience: In addition to adverse events reported from clinical trials, the following events have been identified during post-approval use of MEPRON. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to MEPRON. Blood and Lymphatic System Disorders: Methemoglobinemia, thrombocytopenia. Immune System Disorders: Hypersensitivity reactions including angioedema, bronchospasm, throat tightness, and urticaria. Eye Disorders: Vortex keratopathy. Gastrointestinal Disorders: Pancreatitis. Hepatobiliary Disorders: Rare cases of hepatitis, and one case of fatal liver failure have been reported with atovaquone usage. Skin and Subcutaneous Tissue Disorders: Erythema multiforme, Stevens-Johnson syndrome, and skin desquamation have been reported in patients receiving multiple drug therapy including atovaquone. Renal and Urinary Disorders: Acute renal impairment. OVERDOSAGE There is no known antidote for atovaquone, and it is currently unknown if atovaquone is dialyzable. The median lethal dose is higher than the maximum oral dose tested in mice and rats (1,825 mg/kg/day). Overdoses up to 31,500 mg of atovaquone have been reported. In 1 such patient who also took an unspecified dose of dapsone, methemoglobinemia occurred. Rash has also been reported after overdose. DOSAGE AND ADMINISTRATION Dosage: Prevention of PCP: Adults and Adolescents (13 to 16 Years): The recommended oral dose is 1,500 mg (10 ml) once daily administered with a meal. 15
23 Treatment of Mild-to-Moderate PCP: Adults and Adolescents (13 to 16 Years): The recommended oral dose is 750 mg (5 ml) administered with meals twice daily for 21 days (total daily dose 1,500 mg). Note: Failure to administer MEPRON Suspension with meals may result in lower plasma atovaquone concentrations and may limit response to therapy (see CLINICAL PHARMACOLOGY and PRECAUTIONS). Administration: Foil Pouch: Open pouch by removing tab at perforation and tear at notch. Take entire contents by mouth. Can be discharged into a dosing spoon or cup or directly into the mouth. Bottle: SHAKE BOTTLE GENTLY BEFORE USING. HOW SUPPLIED MEPRON Suspension (bright yellow, citrus flavored) containing 750 mg atovaquone in each teaspoonful (5 ml). Bottle of 210 ml with child-resistant cap (NDC ). Store at 15 to 25 C (59 to 77 F). DO NOT FREEZE. Dispense in tight container as defined in USP. 5-mL child-resistant foil pouch - unit dose pack of 42 (NDC ). Store at 15 to 25 C (59 to 77 F). DO NOT FREEZE. 1 (A-a)DO 2 = [(713 x FiO 2 ) (PaCO 2/0.8)] PaO 2 (mm Hg) GlaxoSmithKline Research Triangle Park, NC , GlaxoSmithKline. All rights reserved. May 2008 MPR:1PI 16
24 MEPRON (atovaquone) 懸濁液 添付文書 性状 MEPRON(atovaquone) は抗原虫剤である 化学名は trans-2-[4-(4- chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione で 水にほとんど溶けない黄色の結晶性固体である 分子量は 分子式は C 22 H 19 ClO 3 で 以下の化学構造を有する MEPRON 懸濁液は atovaquone の超微粒子製剤である 吸収を高めるために小さくした atovaquone の粒子は すでに市販されている錠剤製剤の粒子よりもはるかに小さい MEPRON 懸濁液は経口用製剤で 色は鮮黄色 味は柑橘系である 小さじ 1 杯 (5 ml) 中に atovaquone 750 mg と添加物としてベンジルアルコール 香料 Poloxamer 188 精製水 サッカリンナトリウムおよびキサンタンガムを含有する 微生物学作用機序 :Atovaquone は hydroxy-1,4-naphthoquinone というユビキノン類似体で 抗ニューモシスティス活性を有する ニューモシスティス カリニに対する作用機序は 完全には解明されていない マラリア原虫属においては チトクロム bc 1 複合体 ( 複合体 III) が作用部位であると思われる ユビキノンを介するミトコンドリア電子伝達鎖にはいくつかの代謝酵素が関係している Atovaquone は電子伝達を阻害することにより これらの酵素を間接的に阻害する このような阻害による代謝への影響として 最終的に核酸および ATP の合成が阻害されると考えられる In Vitro での活性 :In vitro でのさまざまな方法を用いたいくつかの検査室において ラットのニューモシスティス カリニに対する atovaquone の IC 50 (50% 阻害濃度 ) は 0.1 ~3.0 mcg/ml であることが確認されている 薬剤耐性 : ニューモシスティス カリニについては in vitro において atovaquone に対する表現型耐性は認められていない しかし atovaquone の予防投与後にニューモシスティス カリニ肺炎 (PCP) を発症した 2 例において DNA 配列解析により ニューモシスティス カリニのチトクロム b(atovaquone の推定標的部位 ) の推定アミノ酸配列に変異が認められた この変異の臨床的意義は不明である 1
25 臨床薬理薬物動態 : 吸収 :Atovaquone は 水に溶けにくい高親油性の化合物である バイオアベイラビリティは製剤や食事の内容によって大きく異なる 本懸濁液製剤では 空腹時または食後の atovaquone のバイオアベイラビリティは すでに市販されている錠剤製剤の約 2 倍となる MEPRON 懸濁液 750 mg を HIV 感染患者 (CD4 が 100 個 /mm 3 超 )9 例に食後投与したときの絶対バイオアベイラビリティは 47±15% であった 同試験において すでに市販されている錠剤製剤 750 mg の投与後のバイオアベイラビリティは 23 ±11% であった Atovaquone を食後に投与すると 吸収率が約 2 倍上昇する ある試験において 健康志願者 16 例に MEPRON 懸濁液 750 mg を一晩絶食後と標準的な朝食 ( 脂肪 23 g 610 kcal) の摂取後にそれぞれ単回投与したところ 濃度時間曲線下面積 (AUC) の平均値 (±SD) は 空腹時で 324±115 hr mcg/ml 食後で 801±320 hr mcg/ml であり 食後では吸収率が 2.6±1.0 倍上昇した また HIV 感染患者 (CD4 が 200 個 /mm 3 未満 ) 19 例に MEPRON 懸濁液 500 mg を連日投与する無作為化反復投与クロスオーバー試験において 食事 ( 脂肪 23 g 400 kcal) による血漿中 atovaquone 濃度への影響も検討した その結果 AUC は 食後投与で 280±114 hr mcg/ml 空腹時投与で 169±77 hr mcg/ml であった 最高血漿中 atovaquone 濃度 (C max ) は 食後投与で 15.1±6.1 空腹時投与で 8.8±3.7 mcg/ml であった 用量比例性 : 血漿中 atovaquone 濃度は用量比例的には上昇しない MEPRON 懸濁液を および 1,000 mg の用量で 1 日 1 回食後投与したときの定常状態の平均血漿中 atovaquone 濃度はそれぞれ 11.7± ±5.8 および 13.5±5.1 mcg/ml であった 対応する C max はそれぞれ 15.1± ±7.6 および 16.8±6.4 mcg/ml であった MEPRON 懸濁液を HIV 感染患者 5 例に 750 mg 1 日 2 回投与したときの定常状態の平均血漿中 atovaquone 濃度は 21.0±4.9 mcg/ml で C max は 24.0±5.7 mcg/ml であった 750 mg を 1 日 2 回投与したときの最低血漿中 atovaquone 濃度 (C min ) は 16.7± 4.6 mcg/ml であった 分布 :Atovaquone の静脈内投与後における定常状態の分布容積 (Vd ss ) は 0.60± 0.17 L/kg(n=9) であった Atovaquone は 1~90 mcg/ml の濃度範囲にわたって高い血漿蛋白結合率 (99.9%) を示す HIV 感染小児 3 例に atovaquone の 750 mg 錠剤製剤を 1 日 4 回 2 週間投与したところ 脳脊髄液中 atovaquone 濃度はそれぞれ および 0.26 mcg/ml で 血漿中濃度の 1% 未満であった 排泄 :HIV 感染患者 9 例における静脈内 (IV) 投与後の atovaquone の血漿クリアランスは 10.4±5.5 ml/min(0.15±0.09 ml/min/kg) であった IV 投与後の atovaquone の半減期は 62.5±35.3 時間で MEPRON 懸濁液を用いた試験全体で 67.0±33.4~77.6±23.1 時間であった Atovaquone は 腸肝循環を経て 最終的に糞中に排泄されるため 半減期が長い 健康志願者に 14 C で標識した atovaquone を投与した試験では 21 日間で投与量の 94% 超が atovaquone の未変化体として糞中に排泄された 尿中にはほとんど あるいはまったく排泄されない (0.6% 未満 ) Atovaquone はわずかに代謝されるということを示す間接的なエビデンスが存在するが 特定の代謝物は同定されていない 特別な集団 : 小児 : 生後 1 ヵ月から 13 歳までの無症候性 HIV 感染乳幼児および小児 27 例を対象とした MEPRON 懸濁液の試験において atovaquone は年齢依存的な薬物動態を 2
26 示した これらの患者には 1 日 1 回 12 週間 食後に投与を行った 濃度データが得られた 24 例における定常状態の平均血漿中 atovaquone 濃度を表 1 に示す 表 1. 小児患者における定常状態の平均血漿中 Atovaquone 濃度 MEPRON 懸濁液の用量 10 mg/kg 30 mg/kg 45 mg/kg 年齢 1 ヵ月超 3 ヵ月以下 5.9 (n = 1) 3 ヵ月超 24 ヵ月以下 5.7 ± 5.1 (n = 4) 2 歳超 13 歳以下 16.8 ± 6.4 (n = 4) 平均 C ss [mcg/ml]( 平均 ± SD) 27.8 ± 5.8 (n = 4) 9.8 ± 3.2 (n = 4) 37.1 ± 10.9 (n = 3) 15.4 ± 6.6 (n = 4) 肝 / 腎機能障害 : 肝 / 腎機能障害を有する患者を対象とした atovaquone の薬物動態試験は これまでに実施されていない 薬物相互作用 : リファンピン :HIV 感染患者 13 例を対象とした試験において リファンピン 600 mg(24 時間ごと ) と MEPRON 懸濁液 750 mg(12 時間ごと ) の併用経口投与を行ったところ 定常状態の平均血漿中 atovaquone 濃度は 52±13% 低下し 定常状態の平均血漿中リファンピン濃度は 37±42% 上昇した Atovaquone の半減期は リファンピン非併用での 82±36 時間から リファンピン併用では 50±16 時間に短縮した 同じくリファマイシン系であるリファブチンは リファンピンと構造が類似しており リファンピンと同様の薬物相互作用を示す可能性がある MEPRON とリファブチンの相互作用試験は これまでに実施されていない トリメトプリム / スルファメトキサゾール (TMP-SMX) 合剤 : 大規模な MEPRON 懸濁液の反復投与 用量漸増 慢性投与試験の一環として HIV 感染成人患者 6 例を対象に atovaquone と TMP-SMX 合剤との相互作用の可能性について検討した このクロスオーバー試験では MEPRON 懸濁液 500 mg 1 日 1 回投与 TMP-SMX 錠 ( トリメトプリム 160 mg とスルファメトキサゾール 800 mg の合剤 )1 日 2 回投与またはこれらの併用投与を定常状態に達するまで食後に行った TMP-SMX 合剤との併用投与では 定常状態の平均血漿中 atovaquone 濃度に差は認められなかった 一方 MEPRON と TMP-SMX 合剤の併用投与では トリメトプリムおよびスルファメトキサゾールの定常状態の平均血漿中濃度がそれぞれ 17% および 8% 低下した この影響はごく小さなものであり 臨床的に問題となる事象を引き起こすことはないと予測される ジドブジン :HIV 感染患者 14 例に atovaquone 錠 750 mg の 12 時間ごと 1 回およびジドブジン 200 mg の 8 時間ごと 1 回の投与を行ったところ ジドブジンの見かけの経口クリアランスが 24±12% 低下し その結果 ジドブジンの血漿中 AUC が 35±23% 増加した グルクロニド代謝物と未変化体の比は ジドブジン単独投与後の平均値である 4.5 から ジドブジンと atovaquone 錠の併用投与後では 3.1 に低下した この影響はごく 3
27 小さなものであり 臨床的に問題となる事象を引き起こすことはないと予測される ジドブジンは atovaquone の薬物動態に影響を及ぼさなかった 血漿中 atovaquone 濃度と臨床転帰の関連性 : 軽度から中等度のニューモシスティス カリニ肺炎 (PCP) の経口治療 ( 効能 効果 の項参照 ) における atovaquone 錠と TMP-SMX 合剤を比較した試験において AIDS 患者に atovaquone 750 mg 錠を 1 日 3 回 21 日間投与したときの定常状態の平均 atovaquone 濃度は 13.9±6.9 mcg/ml(n=133 例 ) であった これらのデータを解析した結果 血漿中 atovaquone 濃度と奏効率との間に関連性が認められた この結果を表 2 に示す 表 2. 血漿中 atovaquone 濃度と奏効率の関連性 定常状態の血漿中 Atovaquone 濃度 奏効率 * ( 奏効例数 / 群内総例数 )(%) (mcg/ml) 実測値 予測値 0~<5 0/6 (0%) 1.5/6 (25%) 5~<10 18/26 (69%) 14.7/26 (57%) 10~<15 30/38 (79%) 31.9/38 (84%) 15~<20 18/19 (95%) 18.1/19 (95%) 20~<25 18/18 (100%) 17.8/18 (99%) 25~ 6/6 (100%) 6/6 (100%) * 奏効とは 臨床的および呼吸指標の改善が投与中止後 4 週間以上持続した状態と定 義した これは 転帰データおよび定常状態の血漿中 atovaquone 濃度のデータのい ずれもが得られた患者のデータに基づく結果である ジスティック回帰解析に基づく 軽度から中等度の PCP の治療のための MEPRON 懸濁液の用量は 平均血漿中 atovaquone 濃度が約 20 mcg/ml に達する用量を選択した それは この血漿中濃度が良好な忍容性を示し 最も高い奏効率をもたらすことがこれまでに確認されているためである ( 表 2) MEPRON 懸濁液を用いた非盲検 PCP 治療試験において 1,000 mg 1 日 1 回 750 mg 1 日 2 回 1,500 mg 1 日 1 回および 1,000 mg 1 日 2 回という用量を探索的に検討した 750 mg 1 日 2 回の食後投与で達した定常状態の平均血漿中 atovaquone 濃度は 22.0±10.1 mcg/ml(n=18) であった 効能 効果 MEPRON 懸濁液は トリメトプリム / スルファメトキサゾール (TMP-SMX) 合剤に不耐容の患者におけるニューモシスティス カリニ肺炎の予防を適応とする その他 MEPRON 懸濁液は TMP-SMX 合剤に不耐容の患者における軽度から中等度の PCP の急性期経口治療を適応とする PCP の予防 :PCP の予防という適応は PCP のリスクがあり (CD4 数が 200 個 /mm 3 未満または PCP 発症の既往歴がある ) TMP-SMX 合剤に不耐容の HIV 感染成人および青年患者を対象として MEPRON 懸濁液をダプソンまたは噴霧用ペンタミジンと比較した 2 件の臨床試験の結果に基づいている 4
28 ダプソンとの比較試験 : この無作為化非盲検試験には 48 施設にて計 1,057 例の患者が組み入れられた これらの患者を MEPRON 懸濁液 1,500 mg 1 日 1 回投与群 (n= 536) またはダプソン 100 mg 1 日 1 回投与群 (n=521) のいずれかに無作為に割り付けた 追跡期間中央値は 24 ヵ月であった ダプソン群に割り付けられた患者のうち トキソプラズマ原虫が血清抗体陽性で CD4 数が 100 個 /mm 3 未満であった患者には ピリメタミンおよびフォリン酸の投与も行った PCP イベントの発生率を表 3 に示す 死亡率に有意な群間差は認められなかった 噴霧用ペンタミジンとの比較試験 : この無作為化非盲検試験には 35 施設にて計 549 例の患者が組み入れられた これらの患者を MEPRON 懸濁液 1,500 mg 1 日 1 回投与群 (n=175) MEPRON 懸濁液 750 mg 1 日 1 回投与群 (n=188) または噴霧用ペンタミジン 300 mg 月 1 回投与群 (n=186) のいずれかに無作為に割り付けた 追跡期間中央値は 11.3 ヵ月であった PCP イベントの発生率を表 3 に示す 死亡率に有意な群間差は認められなかった 表 3. PCP が確定診断または推定診断された / 疑われたイベントの発生率 (As-Treated 解析 )* 試験 試験 評価項目 Atovaquone 1,500 mg/ 日 (n = 527) ダプソン 100 mg/ 日 (n = 510) Atovaquone 750 mg/ 日 (n = 188) Atovaquone 1,500 mg/ 日 (n = 172) 噴霧用ペンタミジン 300 mg/ 月 (n = 169) % 15% 19% 23% 18% 17% 相対リスク (CI) 0.77 (0.57, 1.04) 1.47 (0.86, 2.50) 1.14 (0.63, 2.06) * 割付け治療中または終了後 30 日以内に発生したイベント 相対リスクが 1 を下回ればatovaquoneの優越性が実証され 1 を上回れば対照薬の優 越性が実証される これらの試験はatovaquoneの対照薬に対する優越性を実証するた めにデザインされた これは実証されなかった 区間の信頼度は ダプソンとの比較試験では 95% ペンタミジンとの比較試験では 97.5% であった 全 PCP イベントの解析 (intent-to-treat 解析 ) の結果は 上述の解析の結果と同様であった PCP の治療 : 軽度から中等度の PCP の治療という適応は 懸濁液製剤と錠剤製剤の薬物動態比較試験 ( 臨床薬理 の項参照 ) および血漿中 atovaquone 濃度と奏効率との関連性を実証した錠剤製剤の臨床的有効性試験の結果に基づいている 軽度から中等度の PCP( 治験実施計画書において 室内気吸入下での肺胞気 動脈血酸素分圧較差 [(A-a)DO 2 ] 1 が 45 mm Hg 以下かつ PaO 2 が 60 mm Hg 以上と定義された ) を有する AIDS 患者を対象として MEPRON を TMP-SMX 合剤と比較した無作為化二重盲検試験の結果と 抗菌薬であるトリメトプリムまたはスルファ剤に不耐容の軽度から中等度の PCP 患者を 5
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, Sc
 A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
日本化学療法学会雑誌第51巻第2号
 piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
 A comparison of abdominal versus vaginal hysterectomy for leiomyoma and adenomyosis Kenji ARAHORI, Hisasi KATAYAMA, Suminori NIOKA Department of Obstetrics and Gnecology, National Maizuru Hospital,Kyoto,
A comparison of abdominal versus vaginal hysterectomy for leiomyoma and adenomyosis Kenji ARAHORI, Hisasi KATAYAMA, Suminori NIOKA Department of Obstetrics and Gnecology, National Maizuru Hospital,Kyoto,
CHEMOTHERAPY Fig. 1 Chemical structure of CXM-AX
 Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
untitled
 1988 2000 2002 2004 2006 2008 IFN Lamivudine Adefovir Entecavir 1 Total number 560 Sex (male/female) 424/136 Age (years)* 38 (15-68) Duration of treatment (weeks)* 26 (1-592) Follow-up time (years) 75(05-21
1988 2000 2002 2004 2006 2008 IFN Lamivudine Adefovir Entecavir 1 Total number 560 Sex (male/female) 424/136 Age (years)* 38 (15-68) Duration of treatment (weeks)* 26 (1-592) Follow-up time (years) 75(05-21
CHEMOTHERAPY Fig. 1 Body weight changes of pregnant mice treated orally with AM- 715 Day of sestation
 CHEMOTHERAPY CHEMOTHERAPY Fig. 1 Body weight changes of pregnant mice treated orally with AM- 715 Day of sestation CHEMOTHERAPY Table 1 Preliminaly test of AM- 715 1): Mean } SD *: Significant difference
CHEMOTHERAPY CHEMOTHERAPY Fig. 1 Body weight changes of pregnant mice treated orally with AM- 715 Day of sestation CHEMOTHERAPY Table 1 Preliminaly test of AM- 715 1): Mean } SD *: Significant difference
Jan THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Table 1. Outline of administering doses, routes and sampling times *: 4 ml/hr/kg Bacillus subtilis
 THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Jan. 1987 Jan. 1987 THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Table 1. Outline of administering doses, routes and sampling times *: 4 ml/hr/kg Bacillus subtilis
THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Jan. 1987 Jan. 1987 THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Table 1. Outline of administering doses, routes and sampling times *: 4 ml/hr/kg Bacillus subtilis
CHEMOTHERAPY APR. 1984
 VOL.32 S-3 CHEMOTHERAPY dihydro-4-oxo-7-(1-piperazinyl)-1, 8-naphthyridine- CHEMOTHERAPY APR. 1984 VOL.32 S-3 CHEMOTHERAPY Table 1 Implantation rates and post- implantation survival rates in females mated
VOL.32 S-3 CHEMOTHERAPY dihydro-4-oxo-7-(1-piperazinyl)-1, 8-naphthyridine- CHEMOTHERAPY APR. 1984 VOL.32 S-3 CHEMOTHERAPY Table 1 Implantation rates and post- implantation survival rates in females mated
 Hirosaki University Repository 妊 娠 可 能 てんかん 女 性 の 治 療 管 理 基 準 設 定 に 関 する 研 Title 究 Author(s) 兼 子, 直 Citation てんかん 治 療 研 究 振 興 財 団 研 究 年 報. 4, 1992, p.24-35 Issue Date 1992 URL http://hdl.handle.net/10129/1905
Hirosaki University Repository 妊 娠 可 能 てんかん 女 性 の 治 療 管 理 基 準 設 定 に 関 する 研 Title 究 Author(s) 兼 子, 直 Citation てんかん 治 療 研 究 振 興 財 団 研 究 年 報. 4, 1992, p.24-35 Issue Date 1992 URL http://hdl.handle.net/10129/1905
988 CHEMOTHERAPY NOV. 1971
 988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
Fig. 1 Chemical structure of DL-8280
 Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
日本化学療法学会雑誌第65巻第4号
 Mycoplasma pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae Key words Mycoplasma pneumoniae Mycoplasma pneumoniae M. pneumoniae I M. pneumoniae M. pneumoniae M. pneumoniae
Mycoplasma pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae Key words Mycoplasma pneumoniae Mycoplasma pneumoniae M. pneumoniae I M. pneumoniae M. pneumoniae M. pneumoniae
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study
 CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
 ON A FEW INFLUENCES OF THE DENTAL CARIES IN THE ELEMENTARY SCHOOL PUPIL BY Teruko KASAKURA, Naonobu IWAI, Sachio TAKADA Department of Hygiene, Nippon Dental College (Director: Prof. T. Niwa) The relationship
ON A FEW INFLUENCES OF THE DENTAL CARIES IN THE ELEMENTARY SCHOOL PUPIL BY Teruko KASAKURA, Naonobu IWAI, Sachio TAKADA Department of Hygiene, Nippon Dental College (Director: Prof. T. Niwa) The relationship
Fig. 1 Chemical structure of KW-1070
 Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
日本化学療法学会雑誌第59巻第5号
 Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date URL
 Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date 1958-01 URL http://hdl.handle.net/2433/111559 Right Type Departmental Bulletin
Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date 1958-01 URL http://hdl.handle.net/2433/111559 Right Type Departmental Bulletin
日本化学療法学会雑誌第61巻第4号
 μ μ μ μ μ μ Key words I β μ Sex Age (years) Height (cm) Evaluation items Table1.Characteristics of patients 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) male 12 8 7 27 female 2 1
μ μ μ μ μ μ Key words I β μ Sex Age (years) Height (cm) Evaluation items Table1.Characteristics of patients 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) male 12 8 7 27 female 2 1
 1) University Group Diabetes Program: A study of hypoglycemic agents on vascular complica- in patients with adult-onset tions diabetes. I. Design, methods and baseline results. Diabetes 19 (suppl. 2):
1) University Group Diabetes Program: A study of hypoglycemic agents on vascular complica- in patients with adult-onset tions diabetes. I. Design, methods and baseline results. Diabetes 19 (suppl. 2):
CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels of mezlocillin
 CHEMOTHERAPY Fig. 2 Urinary excretion of mezlocillin Fig. 1 Blood levels of mezlocillin CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels
CHEMOTHERAPY Fig. 2 Urinary excretion of mezlocillin Fig. 1 Blood levels of mezlocillin CHEMOTHERAPY Table 1 Urinary excretion of mezlocillin Fig. 4 Urinary excretion of mezlocillin Fig. 3 Blood levels
 Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
CHEMOTHERAPY FEB Table 1. Activity of cefpirome and others against clinical isolates
 VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
VOL.35 S-2 CHEMOTHERAPY Table 1 Sex and age distribution Table 2 Applications of treatment with carumonam Table 3 Concentration of carumonam in human
 CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
 Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
VOL.39 S-3
 VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
Studies of Foot Form for Footwear Design (Part 9) : Characteristics of the Foot Form of Young and Elder Women Based on their Sizes of Ball Joint Girth
 Studies of Foot Form for Footwear Design (Part 9) : Characteristics of the Foot Form of Young and Elder Women Based on their Sizes of Ball Joint Girth and Foot Breadth Akiko Yamamoto Fukuoka Women's University,
Studies of Foot Form for Footwear Design (Part 9) : Characteristics of the Foot Form of Young and Elder Women Based on their Sizes of Ball Joint Girth and Foot Breadth Akiko Yamamoto Fukuoka Women's University,
CHEMOTHERAPY APR Fig. 2 The inactivation of aminoglycoside antibiotics by PC-904 Fig. 3 Serum concentration of PC-904 (1) Fig. 4 Urinary recover
 VOL.26 S-2 CHEMOTHERAPY Gentamicin (GM), Dibekacin (DKB), Tobramycin Fig. 1 Protein concentration and protein binding rate Table 2 Protein binding rate of PC-904 in serum of healthy adults, and patients
VOL.26 S-2 CHEMOTHERAPY Gentamicin (GM), Dibekacin (DKB), Tobramycin Fig. 1 Protein concentration and protein binding rate Table 2 Protein binding rate of PC-904 in serum of healthy adults, and patients
 CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
 ;~ (Summary) The Study on the Effects of Foot Bathing on Urination Kumiko Toyoda School of Human Nursing, University of Shiga Prefecture Background Foot bathing is one of the important nursing care for
;~ (Summary) The Study on the Effects of Foot Bathing on Urination Kumiko Toyoda School of Human Nursing, University of Shiga Prefecture Background Foot bathing is one of the important nursing care for
 Background : There have not been any informations available of whether diuretics may enhance renoprotective effects of ACEI and ARB. Methods Post-hoc analysis of the results of COOPERATE trial, asking
Background : There have not been any informations available of whether diuretics may enhance renoprotective effects of ACEI and ARB. Methods Post-hoc analysis of the results of COOPERATE trial, asking
 1) Lysozyme and Viral Infections, 2nd Intern- Symposium on Fleming's Lysozyme, ational Milano, Apr. 1961. 2) Ermol'eva, Z.V. et al.: Experimental study and clinical application of lysozyme. Fed. Proc.
1) Lysozyme and Viral Infections, 2nd Intern- Symposium on Fleming's Lysozyme, ational Milano, Apr. 1961. 2) Ermol'eva, Z.V. et al.: Experimental study and clinical application of lysozyme. Fed. Proc.
CHEMOTHERAPY FEB Table 1 Background of volunteers
 CHEMOTHERAPY FEB. 1985 Table 1 Background of volunteers Table 3 Reproducibility of saisomicln In the EIA and the RIA Fig.1 Comparison of the EIA with the RIA or bioassay of sisomicin Table 4 Blood levels
CHEMOTHERAPY FEB. 1985 Table 1 Background of volunteers Table 3 Reproducibility of saisomicln In the EIA and the RIA Fig.1 Comparison of the EIA with the RIA or bioassay of sisomicin Table 4 Blood levels
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S.
 Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
Title 外傷性脊髄損傷患者の泌尿器科学的研究第 3 報 : 上部尿路のレ線学的研究並びに腎機能について Author(s) 伊藤, 順勉 Citation 泌尿器科紀要 (1965), 11(4): Issue Date URL
 Title 外傷性脊髄損傷患者の泌尿器科学的研究第 3 報 : 上部尿路のレ線学的研究並びに腎機能について Author(s) 伊藤, 順勉 Citation 泌尿器科紀要 (1965), 11(4): 278-291 Issue Date 1965-04 URL http://hdl.handle.net/2433/112732 Right Type Departmental Bulletin Paper
Title 外傷性脊髄損傷患者の泌尿器科学的研究第 3 報 : 上部尿路のレ線学的研究並びに腎機能について Author(s) 伊藤, 順勉 Citation 泌尿器科紀要 (1965), 11(4): 278-291 Issue Date 1965-04 URL http://hdl.handle.net/2433/112732 Right Type Departmental Bulletin Paper
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST
 Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
VOL. 36 S-3 CHEMOTHERAPY 437
 VOL. 36 S-3 CHEMOTHERAPY 437 438 CHEMOTHERAPY JULY 1988 Fig. 1 Contractile response of gastrointestinal tract to intravenous administration of saline and EM in interdigestive state in dogs (a) : Saline,
VOL. 36 S-3 CHEMOTHERAPY 437 438 CHEMOTHERAPY JULY 1988 Fig. 1 Contractile response of gastrointestinal tract to intravenous administration of saline and EM in interdigestive state in dogs (a) : Saline,
VOL. 34 S-2 CHEMOTH8RAPY 913
 VOL. 34 S-2 CHEMOTH8RAPY 913 914 CHEMOTHERAPY APR. 1986 Fig. 1 Chemical structure of T-2588 and T-2525 T- 2588 pivaloyloxymethyl (+ )- (6 R, 7 R)-7-[(Z)-2- (2-amino- 4-thiazolyl)-2-methox yiminoacetamido]-3-[(
VOL. 34 S-2 CHEMOTH8RAPY 913 914 CHEMOTHERAPY APR. 1986 Fig. 1 Chemical structure of T-2588 and T-2525 T- 2588 pivaloyloxymethyl (+ )- (6 R, 7 R)-7-[(Z)-2- (2-amino- 4-thiazolyl)-2-methox yiminoacetamido]-3-[(
Experimental and Clinical Studies of Pregnant Hypertension Takashi SHIMAZU Department of Obstetrics and Gynecology, Osaka City University Medical Scho
 Experimental and Clinical Studies of Pregnant Hypertension Takashi SHIMAZU Department of Obstetrics and Gynecology, Osaka City University Medical School While the problem of late pregnant hypertension
Experimental and Clinical Studies of Pregnant Hypertension Takashi SHIMAZU Department of Obstetrics and Gynecology, Osaka City University Medical School While the problem of late pregnant hypertension
 rectomy as the subjects of study, urinary steroid hormones were measured, and simultaneously the enzyme work and enzyme pattern related to the steroid metabolism, as proposed by Yoshida of our laboratory,
rectomy as the subjects of study, urinary steroid hormones were measured, and simultaneously the enzyme work and enzyme pattern related to the steroid metabolism, as proposed by Yoshida of our laboratory,
VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity of gram nega
 1066 CHEMOTHERAPY MAR. 1975 Table 1 Sensitivity of standard strains VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity
1066 CHEMOTHERAPY MAR. 1975 Table 1 Sensitivity of standard strains VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity
Key words: Antibodies to Leptospira, Tokyo, Uveitis
 Key words: Antibodies to Leptospira, Tokyo, Uveitis Fig. 1 Distribution of Antibody Titers in Age Decade Fig. 3 Distribution of Antibody Titers in each Strain Fig. 2 Correlation between Antibody Titers
Key words: Antibodies to Leptospira, Tokyo, Uveitis Fig. 1 Distribution of Antibody Titers in Age Decade Fig. 3 Distribution of Antibody Titers in each Strain Fig. 2 Correlation between Antibody Titers
 EVALUATION OF NOCTURNAL PENILE TUMESCENCE (NPT) IN THE DIFFERENTIAL DIAGNOSIS OF IMPOTENCE Masaharu Aoki, Yoshiaki Kumamoto, Kazutomi Mohri and Kazunori Ohno Department of Urology, Sapporo Medical College
EVALUATION OF NOCTURNAL PENILE TUMESCENCE (NPT) IN THE DIFFERENTIAL DIAGNOSIS OF IMPOTENCE Masaharu Aoki, Yoshiaki Kumamoto, Kazutomi Mohri and Kazunori Ohno Department of Urology, Sapporo Medical College
Title 歯性病巣の関連する皮膚疾患におけるビオチンの効用 Author(s) 高橋, 愼一 ; 川島, 淳子 ; 森本, 光明 ; 山根, 源之 Journal, (): - URL Right Posted at the Inst
 Title 歯性病巣の関連する皮膚疾患におけるビオチンの効用 Author(s) 高橋, 愼一 ; 川島, 淳子 ; 森本, 光明 ; 山根, 源之 Journal, (): - URL http://hdl.handle.net/10130/526 Right Posted at the Institutional Resources for Unique Colle Available from
Title 歯性病巣の関連する皮膚疾患におけるビオチンの効用 Author(s) 高橋, 愼一 ; 川島, 淳子 ; 森本, 光明 ; 山根, 源之 Journal, (): - URL http://hdl.handle.net/10130/526 Right Posted at the Institutional Resources for Unique Colle Available from
スライド 1
 医薬品副作用報告 中部ろうさい病院薬剤部 2016 年 4 月 1 日 1 中部ろうさい病院副作用報告の書式について 下記ダウンロードページにアクセスしていただき 必要なファイルをダウンロードしてください 書式のファイルは 副作用報告 のリンク先のページに置いてあります ダウンロードページURL http://www.chubuh.johas.go.jp/clinicaltrial/database/
医薬品副作用報告 中部ろうさい病院薬剤部 2016 年 4 月 1 日 1 中部ろうさい病院副作用報告の書式について 下記ダウンロードページにアクセスしていただき 必要なファイルをダウンロードしてください 書式のファイルは 副作用報告 のリンク先のページに置いてあります ダウンロードページURL http://www.chubuh.johas.go.jp/clinicaltrial/database/
Visual Evaluation of Polka-dot Patterns Yoojin LEE and Nobuko NARUSE * Granduate School of Bunka Women's University, and * Faculty of Fashion Science,
 Visual Evaluation of Polka-dot Patterns Yoojin LEE and Nobuko NARUSE * Granduate School of Bunka Women's University, and * Faculty of Fashion Science, Bunka Women's University, Shibuya-ku, Tokyo 151-8523
Visual Evaluation of Polka-dot Patterns Yoojin LEE and Nobuko NARUSE * Granduate School of Bunka Women's University, and * Faculty of Fashion Science, Bunka Women's University, Shibuya-ku, Tokyo 151-8523
untitled
 () 2006 i Foundationpowdermakeup No.1 ii iii iv Research on selection criterion of cosmetics that use the consumer's Eras analysis Consideration change by bringing up child Fukuda Eri 1.Background, purpose,
() 2006 i Foundationpowdermakeup No.1 ii iii iv Research on selection criterion of cosmetics that use the consumer's Eras analysis Consideration change by bringing up child Fukuda Eri 1.Background, purpose,
 Fig. 1 Mean body weight changes of male rats administered cefaclor orally on fertility study Table. 1 Serum biochemical analysis in male rats administered cefaclor orally on fertility study The values
Fig. 1 Mean body weight changes of male rats administered cefaclor orally on fertility study Table. 1 Serum biochemical analysis in male rats administered cefaclor orally on fertility study The values
過去26年間のスギ花粉飛散パターンのクラスター分析
 117 681 : 2A 2B 2C 2A 2B 2C 2A 2A 2B 2C 2A 2B 2C 2A : DNA Phöbus Blackly 1cm 117 682 2014 1 SYSTAT χ Complete linkage method χ 2A 2B 2C /cm /cm /cm 2A 2B 2C 2A 2B 2C 2A 2B 2C 2 A /cm 2A 2C 117 683 2 2A
117 681 : 2A 2B 2C 2A 2B 2C 2A 2A 2B 2C 2A 2B 2C 2A : DNA Phöbus Blackly 1cm 117 682 2014 1 SYSTAT χ Complete linkage method χ 2A 2B 2C /cm /cm /cm 2A 2B 2C 2A 2B 2C 2A 2B 2C 2 A /cm 2A 2C 117 683 2 2A
Phase II clinical trials for patients with cancer
 II II I 1 I III 14 20 14 20 14 0.05 14 20 1 10 25 20-80 100-200 95 CI (π B π A ) 1.96 [π A (1 π A )/n A + π B (1 π B )/n B ] π A = probability of response rate by treatment A π B = probability of response
II II I 1 I III 14 20 14 20 14 0.05 14 20 1 10 25 20-80 100-200 95 CI (π B π A ) 1.96 [π A (1 π A )/n A + π B (1 π B )/n B ] π A = probability of response rate by treatment A π B = probability of response
Therapy for Asthenopia in Cases of Convergence Insufficiency Hiroko TAKASAKI, C.O.J., Nobuko INAGAMI, C.O.J., and Kayoko TAKENAWA, C.O.J.. Orthoptic c
 Therapy for Asthenopia in Cases of Convergence Insufficiency Hiroko TAKASAKI, C.O.J., Nobuko INAGAMI, C.O.J., and Kayoko TAKENAWA, C.O.J.. Orthoptic clinic, Department of Ophthalmology, (Director: Prof
Therapy for Asthenopia in Cases of Convergence Insufficiency Hiroko TAKASAKI, C.O.J., Nobuko INAGAMI, C.O.J., and Kayoko TAKENAWA, C.O.J.. Orthoptic clinic, Department of Ophthalmology, (Director: Prof
2 The Bulletin of Meiji University of Integrative Medicine 3, Yamashita 10 11
 1-122013 1 2 1 2 20 2,000 2009 12 1 2 1,362 68.1 2009 1 1 9.5 1 2.2 3.6 0.82.9 1.0 0.2 2 4 3 1 2 4 3 Key words acupuncture and moxibustion Treatment with acupuncture, moxibustion and Anma-Massage-Shiatsu
1-122013 1 2 1 2 20 2,000 2009 12 1 2 1,362 68.1 2009 1 1 9.5 1 2.2 3.6 0.82.9 1.0 0.2 2 4 3 1 2 4 3 Key words acupuncture and moxibustion Treatment with acupuncture, moxibustion and Anma-Massage-Shiatsu
CHEMOTHERAPY aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Prote
 aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Proteus mirabilis 3.13, Proteus vulgaris 1.56, Citrobacter freundii 0.39,
aureus 0.10, Enterococcus faecalis 3.13, Escherichia coli 0.20, Klebsiella pneumoniae, Enterobacter spp., Serratia marcescens 0.78, Proteus mirabilis 3.13, Proteus vulgaris 1.56, Citrobacter freundii 0.39,
明海大学歯学雑誌 37‐2/1.秦泉寺
 J Meikai Dent Med 37, 153 158, 8 153 1 7 5ml /1 min Wong-Baker Face Rating Scale FS 5 1 9. g 3 5ml /1 min, FS 1 66 6ml /1 min FS 5 1 9. g 3 6ml /1 min, FS 3 Two Cases of Xerostomia that Showed an Improvement
J Meikai Dent Med 37, 153 158, 8 153 1 7 5ml /1 min Wong-Baker Face Rating Scale FS 5 1 9. g 3 5ml /1 min, FS 1 66 6ml /1 min FS 5 1 9. g 3 6ml /1 min, FS 3 Two Cases of Xerostomia that Showed an Improvement
広島県獣医学会雑誌24号.indd
 1 1 Study on the effects of dexamethasone administration in acute coccidiosis accompanied by bloody stools TOMOYASU KUROSE Shoubara Veterinary Clinical Center, P. F. A. M. A. A -1-, Nishihonmachi, Shoubara,
1 1 Study on the effects of dexamethasone administration in acute coccidiosis accompanied by bloody stools TOMOYASU KUROSE Shoubara Veterinary Clinical Center, P. F. A. M. A. A -1-, Nishihonmachi, Shoubara,
 VOL.32 S-9 CHEMOTHERAPY Table 1 Minimum inhibitory concentrations of AC-1370, CPZ and CAZ Table 2 Efficacy of AC-1370 and CPZ against systemic infections in mice *Inoculum size: 106 cells/ml * 95% confidence
VOL.32 S-9 CHEMOTHERAPY Table 1 Minimum inhibitory concentrations of AC-1370, CPZ and CAZ Table 2 Efficacy of AC-1370 and CPZ against systemic infections in mice *Inoculum size: 106 cells/ml * 95% confidence
Huawei G6-L22 QSG-V100R001_02
 G6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 1 2 3 17 4 5 18 UI 100% 8:08 19 100% 8:08 20 100% 8:08 21 100% 8:08 22 100% 8:08 ********** 23 100% 8:08 Happy birthday! 24 S S 25 100% 8:08 26 http://consumer.huawei.com/jp/
G6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 1 2 3 17 4 5 18 UI 100% 8:08 19 100% 8:08 20 100% 8:08 21 100% 8:08 22 100% 8:08 ********** 23 100% 8:08 Happy birthday! 24 S S 25 100% 8:08 26 http://consumer.huawei.com/jp/
™…
 Review The Secret to Healthy Long Life Decrease in Oxidative and Mental Stress My motto is Health is not all. But nothing can be done without health. Health is the most important requisite for all human
Review The Secret to Healthy Long Life Decrease in Oxidative and Mental Stress My motto is Health is not all. But nothing can be done without health. Health is the most important requisite for all human
 Title 生活年令による学級の等質化に関する研究 (1) - 生活年令と学業成績について - Author(s) 与那嶺, 松助 ; 東江, 康治 Citation 研究集録 (5): 33-47 Issue Date 1961-12 URL http://hdl.handle.net/20.500.12000/ Rights 46 STUDIES ON HOMOGENEOUS
Title 生活年令による学級の等質化に関する研究 (1) - 生活年令と学業成績について - Author(s) 与那嶺, 松助 ; 東江, 康治 Citation 研究集録 (5): 33-47 Issue Date 1961-12 URL http://hdl.handle.net/20.500.12000/ Rights 46 STUDIES ON HOMOGENEOUS
Vol. 36, Special Issue, S 3 S 18 (2015) PK Phase I Introduction to Pharmacokinetic Analysis Focus on Phase I Study 1 2 Kazuro Ikawa 1 and Jun Tanaka 2
 Vol. 36, Special Issue, S 3 S 18 (2015) PK Phase I Introduction to Pharmacokinetic Analysis Focus on Phase I Study 1 2 Kazuro Ikawa 1 and Jun Tanaka 2 1 2 1 Department of Clinical Pharmacotherapy, Hiroshima
Vol. 36, Special Issue, S 3 S 18 (2015) PK Phase I Introduction to Pharmacokinetic Analysis Focus on Phase I Study 1 2 Kazuro Ikawa 1 and Jun Tanaka 2 1 2 1 Department of Clinical Pharmacotherapy, Hiroshima
Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone
 Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone (inoculum size= 106 CFU/ml) (Ĉ-lactamase producer : 2 strains) Fig. 1. Sensitivity distribution of
Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone (inoculum size= 106 CFU/ml) (Ĉ-lactamase producer : 2 strains) Fig. 1. Sensitivity distribution of
Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-
 Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl) oxy]-14-ethyl-12,
Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl) oxy]-14-ethyl-12,
Title 神経因性膀胱にともなう排尿障害に対する塩酸ブナゾシンの臨床効果 - 二重盲検比較試験による検討 - 小柳, 知彦 ; 富樫, 正樹 ; 丸, 彰夫 ; 折笠, 精一 ; 相馬, Author(s) 崎, 淳 ; 安田, 耕作 ; 阿曽, 佳郎 ; 本問, 之夫 ; 三宅, 弘厚生 ; 熊
 Title 神経因性膀胱にともなう排尿障害に対する塩酸ブナゾシンの臨床効果 - 二重盲検比較試験による検討 - 小柳, 知彦 ; 富樫, 正樹 ; 丸, 彰夫 ; 折笠, 精一 ; 相馬, Author(s) 崎, 淳 ; 安田, 耕作 ; 阿曽, 佳郎 ; 本問, 之夫 ; 三宅, 弘厚生 ; 熊澤, 淨一 ; 武井, 実根雄 Citation 泌尿器科紀要 (1990), 36(10): 1233-1252
Title 神経因性膀胱にともなう排尿障害に対する塩酸ブナゾシンの臨床効果 - 二重盲検比較試験による検討 - 小柳, 知彦 ; 富樫, 正樹 ; 丸, 彰夫 ; 折笠, 精一 ; 相馬, Author(s) 崎, 淳 ; 安田, 耕作 ; 阿曽, 佳郎 ; 本問, 之夫 ; 三宅, 弘厚生 ; 熊澤, 淨一 ; 武井, 実根雄 Citation 泌尿器科紀要 (1990), 36(10): 1233-1252
untitled
 4 2008 1 250mg JAN Metformin HydrochlorideJAN Goat s ruegalega officinalis L. 1918 1950 1970 1990 United Kingdom Prospective Diabetes StudyUKPDS 1961 1993 3 2006 8 250 mg 2007 12 SU 2 2 11 2 1 4 104
4 2008 1 250mg JAN Metformin HydrochlorideJAN Goat s ruegalega officinalis L. 1918 1950 1970 1990 United Kingdom Prospective Diabetes StudyUKPDS 1961 1993 3 2006 8 250 mg 2007 12 SU 2 2 11 2 1 4 104
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates
 Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
 b) Gram-negative bacteria Fig. 2 Sensitivity distribution of clinical isolates : E. coli Fig. 3 Sensitivity distribution of clinical isolates : Pseudomonas Fig. 1 Sensitivity distribution of clinical isolates
b) Gram-negative bacteria Fig. 2 Sensitivity distribution of clinical isolates : E. coli Fig. 3 Sensitivity distribution of clinical isolates : Pseudomonas Fig. 1 Sensitivity distribution of clinical isolates
 44 2012 2013 3 35 48 法人化後の国立大学の収入変動 37 法人化後の国立大学の収入変動 2009 2005 2010 2012 2012 2008 2009a 2010 16 18 17 20 2 4 2012 38 44 2012 17 22 (1) (2) 2012 5 GP COE 30 WPI 1 2012 17 22 16 17 22 17 17 19 2012 2012
44 2012 2013 3 35 48 法人化後の国立大学の収入変動 37 法人化後の国立大学の収入変動 2009 2005 2010 2012 2012 2008 2009a 2010 16 18 17 20 2 4 2012 38 44 2012 17 22 (1) (2) 2012 5 GP COE 30 WPI 1 2012 17 22 16 17 22 17 17 19 2012 2012
208 ( 2 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 63 _ 3 June 2010 Cefditoren pivoxil (CDTR-PI) MS MS 10%
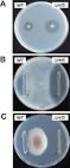 207 ( 1 ) Cefditoren pivoxil G 2010 2 2 2006 3 Cefditoren pivoxil CDTR-PI MS 10% CDTR-PI 305 2,144 2,006 1,958 1.79% 36 2,006 26 (1.30%) CDTR-PI CDTR-PI (9 mg/kg/day) 1.5 2 (2.70%) 2 (1.92%) 93.5% 1,831
207 ( 1 ) Cefditoren pivoxil G 2010 2 2 2006 3 Cefditoren pivoxil CDTR-PI MS 10% CDTR-PI 305 2,144 2,006 1,958 1.79% 36 2,006 26 (1.30%) CDTR-PI CDTR-PI (9 mg/kg/day) 1.5 2 (2.70%) 2 (1.92%) 93.5% 1,831
2 94
 32 2008 pp. 93 106 1 Received October 30, 2008 The purpose of this study is to examine the effects of aerobics training class on weight loss for female students in HOKURIKU UNIVERSITY. Seventy four female
32 2008 pp. 93 106 1 Received October 30, 2008 The purpose of this study is to examine the effects of aerobics training class on weight loss for female students in HOKURIKU UNIVERSITY. Seventy four female
STUDIES ON THE RELATION BETWEEN LATE DUMPING SYNDROME AND GLUCAGON RESPONCES TO GLUCOSE Taisuke MATSUI The First Department of Surgery, Nara Medical U
 STUDIES ON THE RELATION BETWEEN LATE DUMPING SYNDROME AND GLUCAGON RESPONCES TO GLUCOSE Taisuke MATSUI The First Department of Surgery, Nara Medical University, Kashihara (Director: Prof. H. Nakano) This
STUDIES ON THE RELATION BETWEEN LATE DUMPING SYNDROME AND GLUCAGON RESPONCES TO GLUCOSE Taisuke MATSUI The First Department of Surgery, Nara Medical University, Kashihara (Director: Prof. H. Nakano) This
1272 CHEMOTHERAPY MAR. 1975
 1272 CHEMOTHERAPY MAR. 1975 VOL. 23 NO. 3 CHEMOTHERAPY 1273 Fig. 2 Minimal inhibitory concentration of aminoglycosides against 50 strains of Klebsiella Fig. 1 Minimal inhibitory concentration of aminoglycosides
1272 CHEMOTHERAPY MAR. 1975 VOL. 23 NO. 3 CHEMOTHERAPY 1273 Fig. 2 Minimal inhibitory concentration of aminoglycosides against 50 strains of Klebsiella Fig. 1 Minimal inhibitory concentration of aminoglycosides
 Title 個人 集団レベルの心理社会的学校環境が生体的ストレス反応に及ぼす影響 Author(s) 高倉, 実 ; 小林, 稔 ; 和氣, 則江 ; 安仁屋, 洋子 Citation Issue Date 2007-03 URL http://hdl.handle.net/20.500.12000/ Rights Abstracts of Research Project, Grant-in-Aid
Title 個人 集団レベルの心理社会的学校環境が生体的ストレス反応に及ぼす影響 Author(s) 高倉, 実 ; 小林, 稔 ; 和氣, 則江 ; 安仁屋, 洋子 Citation Issue Date 2007-03 URL http://hdl.handle.net/20.500.12000/ Rights Abstracts of Research Project, Grant-in-Aid
 Influences of mortality from main causes of death on life expectancy. \ An observation for the past 25 years, 1950-1975, in Japan \ Takao SHIGEMATSU* and Zenji NANJO** With the Keyfitz-Nanjo method an
Influences of mortality from main causes of death on life expectancy. \ An observation for the past 25 years, 1950-1975, in Japan \ Takao SHIGEMATSU* and Zenji NANJO** With the Keyfitz-Nanjo method an
 Table 1 Sensitivity distribution of clinical isolates 1. Escherichia coli Inoculum size: 106cells/ml 2. Klebsiella pneumoniae 3. Enterobacter cloacae 4. Serratia marcescens Inoculum size: 106cells/nil
Table 1 Sensitivity distribution of clinical isolates 1. Escherichia coli Inoculum size: 106cells/ml 2. Klebsiella pneumoniae 3. Enterobacter cloacae 4. Serratia marcescens Inoculum size: 106cells/nil
 The Nutrient Intake of Young Women With Special Attention on the Trace Nutrients Including Folate SUZUKI Kazu', HIGASHINE Yuuko", ITOU Ryoko YAMADA Yukiko', KOSEKI Sakiyo*, OKUDA Toyoko"" Seibo Jogakuin
The Nutrient Intake of Young Women With Special Attention on the Trace Nutrients Including Folate SUZUKI Kazu', HIGASHINE Yuuko", ITOU Ryoko YAMADA Yukiko', KOSEKI Sakiyo*, OKUDA Toyoko"" Seibo Jogakuin
 19 OUR EXPERIENCE IN COMBINED BALNEO AND CHRYSOTHERAPY FOR RHEUMATOID ARTHRITIS by Hidemitsu EZAWA (Director: Prof. Hiroshi MORI NAG A), Department ofinternal Medicine, Institute for Thermal Spring Research,
19 OUR EXPERIENCE IN COMBINED BALNEO AND CHRYSOTHERAPY FOR RHEUMATOID ARTHRITIS by Hidemitsu EZAWA (Director: Prof. Hiroshi MORI NAG A), Department ofinternal Medicine, Institute for Thermal Spring Research,
VOL. 23 NO. 3 CHEMOTHERAPY 1379 Table 1 Susceptibility of clinical isolated strains to Tobramycin
 VOL. 23 NO. 3 CHEMOTHERAPY 1379 Table 1 Susceptibility of clinical isolated strains to Tobramycin 1380 CHEMOTHERAPY MAR. 1975 Table 2 Susceptibility of isolated Pseudomonas aeruginosa to various antibiotics
VOL. 23 NO. 3 CHEMOTHERAPY 1379 Table 1 Susceptibility of clinical isolated strains to Tobramycin 1380 CHEMOTHERAPY MAR. 1975 Table 2 Susceptibility of isolated Pseudomonas aeruginosa to various antibiotics
Physical and Psychological Effects of Stressors in Female College Students Reizou Mita*1, Konosuke Tomabechi*1, Isao Yamaguchi*1, Naoko Soeno*1, Shuhe
 Physical and Psychological Effects of Stressors in Female College Students Reizou Mita*1, Konosuke Tomabechi*1, Isao Yamaguchi*1, Naoko Soeno*1, Shuhei Kobayashi*2, Mamoru Nishimuta*2, Michiyuki Shimizu*3,
Physical and Psychological Effects of Stressors in Female College Students Reizou Mita*1, Konosuke Tomabechi*1, Isao Yamaguchi*1, Naoko Soeno*1, Shuhei Kobayashi*2, Mamoru Nishimuta*2, Michiyuki Shimizu*3,
220 28;29) 30 35) 26;27) % 8.0% 9 36) 8) 14) 37) O O 13 2 E S % % 2 6 1fl 2fl 3fl 3 4
 Vol. 12 No. 2 2002 219 239 Λ1 Λ1 729 1 2 29 4 3 4 5 1) 2) 3) 4 6) 7 27) Λ1 701-0193 288 219 220 28;29) 30 35) 26;27) 0 6 7 12 13 18 59.9% 8.0% 9 36) 8) 14) 37) 1 1 1 13 6 7 O O 13 2 E S 1 1 17 0 6 1 585
Vol. 12 No. 2 2002 219 239 Λ1 Λ1 729 1 2 29 4 3 4 5 1) 2) 3) 4 6) 7 27) Λ1 701-0193 288 219 220 28;29) 30 35) 26;27) 0 6 7 12 13 18 59.9% 8.0% 9 36) 8) 14) 37) 1 1 1 13 6 7 O O 13 2 E S 1 1 17 0 6 1 585
coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, F
 VOL.43 S-1 coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, Flavobacter- Table 1. Concentration of grepafloxacin
VOL.43 S-1 coccus aureus Corynebacterium sp, Haemophilus parainfluenzae Klebsiella pneumoniae Pseudornonas aeruginosa Pseudomonas sp., Xanthomonas maltophilia, Flavobacter- Table 1. Concentration of grepafloxacin
Fig. 1 Chemical structure of norfioxacin (AM-715)
 Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
 220 INTERRELATIONSHIPS AMONG TYPE OF REINFORCEMENT, ANXIETY, GSR, AND VERBAL CONDITIONING Koji Tamase Department of Psychology, Nara University of Education, Nara, Japan This investigation examined the
220 INTERRELATIONSHIPS AMONG TYPE OF REINFORCEMENT, ANXIETY, GSR, AND VERBAL CONDITIONING Koji Tamase Department of Psychology, Nara University of Education, Nara, Japan This investigation examined the
CHEMOTHERAPY MAY 1988
 VOL. 36 NO. 5 Table 1. Subjects studied B. W. Body weight B. H. Body height Abbreviations Ccr Endogenous creatinine clearance PSP Phenolsulfonphthalein test for 15 min BUN Blood urea nitrogen Scr Serum
VOL. 36 NO. 5 Table 1. Subjects studied B. W. Body weight B. H. Body height Abbreviations Ccr Endogenous creatinine clearance PSP Phenolsulfonphthalein test for 15 min BUN Blood urea nitrogen Scr Serum
Validation of a Food Frequency Questionnaire Based on Food Groups for Estimating Individual Nutrient Intake Keiko Takahashi *', Yukio Yoshimura *', Ta
 Validation of a Food Frequency Questionnaire Based on Food Groups for Estimating Individual Nutrient Intake Keiko Takahashi *', Yukio Yoshimura *', Tae Kaimoto *', Daisuke Kunii *', Tatsushi Komatsu *2
Validation of a Food Frequency Questionnaire Based on Food Groups for Estimating Individual Nutrient Intake Keiko Takahashi *', Yukio Yoshimura *', Tae Kaimoto *', Daisuke Kunii *', Tatsushi Komatsu *2
 Table 1 Survival rates of infected mice given antibiotic doses producing peak serum a) S. aurcus Smith Challenge dose :7 ~10 (5% mucin) CFU/mouse. LD50: 1 ~103 (5% mucin) CFU/mouse. Table 2 Survival rates
Table 1 Survival rates of infected mice given antibiotic doses producing peak serum a) S. aurcus Smith Challenge dose :7 ~10 (5% mucin) CFU/mouse. LD50: 1 ~103 (5% mucin) CFU/mouse. Table 2 Survival rates
日本職業・災害医学会会誌第51巻第1号
 内野ら 下腿骨骨折におけるハイブリッド創外固定器の有用性 31 図 2 軟部組織損傷程度と機能成績 Gustilo IIIB 以上の開放骨折では Gustilo IIIA 以下の開放骨折 及び皮下骨折よりも明らかに機能低下が認められた 図 1 年齢と機能成績 60 歳未満の若年者の方が明らかに良好な成績を示した a b c 図 3 a Gustilo Type IIIB b, c AO 分類 Type
内野ら 下腿骨骨折におけるハイブリッド創外固定器の有用性 31 図 2 軟部組織損傷程度と機能成績 Gustilo IIIB 以上の開放骨折では Gustilo IIIA 以下の開放骨折 及び皮下骨折よりも明らかに機能低下が認められた 図 1 年齢と機能成績 60 歳未満の若年者の方が明らかに良好な成績を示した a b c 図 3 a Gustilo Type IIIB b, c AO 分類 Type
A5 PDF.pwd
 DV DV DV DV DV DV 67 1 2016 5 383 DV DV DV DV DV DV DV DV DV 384 67 1 2016 5 DV DV DV NPO DV NPO NPO 67 1 2016 5 385 DV DV DV 386 67 1 2016 5 DV DV DV DV DV WHO Edleson, J. L. 1999. The overlap between
DV DV DV DV DV DV 67 1 2016 5 383 DV DV DV DV DV DV DV DV DV 384 67 1 2016 5 DV DV DV NPO DV NPO NPO 67 1 2016 5 385 DV DV DV 386 67 1 2016 5 DV DV DV DV DV WHO Edleson, J. L. 1999. The overlap between
CHEMOTHERAPY SEPT. 1991
 CHEMOTHERAPY SEPT. 1991 VOL. 39 S-3 Table 1. Background of characteristics and allocation of 9 healthy male volunteers in a single-dose study on betamipron Table 2. Background of characteristics and allocation
CHEMOTHERAPY SEPT. 1991 VOL. 39 S-3 Table 1. Background of characteristics and allocation of 9 healthy male volunteers in a single-dose study on betamipron Table 2. Background of characteristics and allocation
indd
 12 61 1 2011 原著 1)2)5) 1) 1)2) 1)2)5) 1)3)5) 1)2) 1)2)5) 1)2) 1)2) 2) 2) 4) 1) 1) 2) 3) 4) 5) 2010 10 6 2010 12 6 A Comparative Study of the Effect and Discomfort Produced by Pharyngeal Anesthesia with
12 61 1 2011 原著 1)2)5) 1) 1)2) 1)2)5) 1)3)5) 1)2) 1)2)5) 1)2) 1)2) 2) 2) 4) 1) 1) 2) 3) 4) 5) 2010 10 6 2010 12 6 A Comparative Study of the Effect and Discomfort Produced by Pharyngeal Anesthesia with
CHEMOTHERAPY JUN Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter cloacae 27, Proteus rettgeri 7, Proteus inconstans 20, Proteus
 VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
The Effect of the Circumferential Temperature Change on the Change in the Strain Energy of Carbon Steel during the Rotatory Bending Fatigue Test by Ch
 The Effect of the Circumferential Temperature Change on the Change in the Strain Energy of Carbon Steel during the Rotatory Bending Fatigue Test by Chikara MINAMISAWA, Nozomu AOKI (Department of Mechanical
The Effect of the Circumferential Temperature Change on the Change in the Strain Energy of Carbon Steel during the Rotatory Bending Fatigue Test by Chikara MINAMISAWA, Nozomu AOKI (Department of Mechanical
Kekkaku Vol. 59, No8 STUDIES ON THE ORIGIN AND CLINICAL SIGNIFICANCE OF SMEAR-POSITIVE AND CULTURE-NEGATIVE TUBERCLE BACILLI Michio TSUKAMURA* and Har
 Kekkaku Vol. 59, No8 STUDIES ON THE ORIGIN AND CLINICAL SIGNIFICANCE OF SMEAR-POSITIVE AND CULTURE-NEGATIVE TUBERCLE BACILLI Michio TSUKAMURA* and Haruo TOYAMA (Received for publication April 4, 1984)
Kekkaku Vol. 59, No8 STUDIES ON THE ORIGIN AND CLINICAL SIGNIFICANCE OF SMEAR-POSITIVE AND CULTURE-NEGATIVE TUBERCLE BACILLI Michio TSUKAMURA* and Haruo TOYAMA (Received for publication April 4, 1984)
48-3 ‰{”R†i74-84†j
 Metallic Stents in the Gastrointestinal Tract Shiro Miyayama, M.D., Tetsuya Komatsu, M.D., Keiichi Taki, M.D., Tetsuya Minami, M.D., Chiharu Ito, M.D., Rieko Shinmura, M.D., Shigeyuki Takamatsu, M.D.,
Metallic Stents in the Gastrointestinal Tract Shiro Miyayama, M.D., Tetsuya Komatsu, M.D., Keiichi Taki, M.D., Tetsuya Minami, M.D., Chiharu Ito, M.D., Rieko Shinmura, M.D., Shigeyuki Takamatsu, M.D.,
 2) Dolphin, A., Jenner, P., Marsden, C,D., Pycock, C. and Tarsy, D. : Pharmacological evidence for cerebral dopamine receptor blockade by metoclopramide in rodents, Psychopharmacologia, 41 : 133, 1975.
2) Dolphin, A., Jenner, P., Marsden, C,D., Pycock, C. and Tarsy, D. : Pharmacological evidence for cerebral dopamine receptor blockade by metoclopramide in rodents, Psychopharmacologia, 41 : 133, 1975.
Table 1 Characteristics of the study participants in Imari municipal hospital
 Key words: tuberculosis, booster phenomenon, two-step tuberculin test Table 1 Characteristics of the study participants in Imari municipal hospital Fig. 1 Frequency distributions of size of the first tuberculin
Key words: tuberculosis, booster phenomenon, two-step tuberculin test Table 1 Characteristics of the study participants in Imari municipal hospital Fig. 1 Frequency distributions of size of the first tuberculin
2 10 The Bulletin of Meiji University of Integrative Medicine 1,2 II 1 Web PubMed elbow pain baseball elbow little leaguer s elbow acupun
 10 1-14 2014 1 2 3 4 2 1 2 3 4 Web PubMed elbow pain baseball elbow little leaguer s elbow acupuncture electric acupuncture 2003 2012 10 39 32 Web PubMed Key words growth stage elbow pain baseball elbow
10 1-14 2014 1 2 3 4 2 1 2 3 4 Web PubMed elbow pain baseball elbow little leaguer s elbow acupuncture electric acupuncture 2003 2012 10 39 32 Web PubMed Key words growth stage elbow pain baseball elbow
Highlights Employment increased in Ontario, Saskatchewan, and Manitoba. There was little change in the other provinces. More people were employed in c
 商工会事務局より (From Shokokai) カナダ統計局より 6 月雇用統計発表 : 失業率 6.0%(+0.2) Labour Force Survey: June Unemployment 6.0%(+0.2) 会員各位 7 月 6 日 カナダ統計局 (Statistics Canada) より 2018 年 6 月の雇用統計が発表になりました 下記概要ポイント仮訳は あくまで商工会事務局で訳したものであり英語の微妙な表現を保証したものではありません
商工会事務局より (From Shokokai) カナダ統計局より 6 月雇用統計発表 : 失業率 6.0%(+0.2) Labour Force Survey: June Unemployment 6.0%(+0.2) 会員各位 7 月 6 日 カナダ統計局 (Statistics Canada) より 2018 年 6 月の雇用統計が発表になりました 下記概要ポイント仮訳は あくまで商工会事務局で訳したものであり英語の微妙な表現を保証したものではありません
L1 What Can You Blood Type Tell Us? Part 1 Can you guess/ my blood type? Well,/ you re very serious person/ so/ I think/ your blood type is A. Wow!/ G
 L1 What Can You Blood Type Tell Us? Part 1 Can you guess/ my blood type? 当ててみて / 私の血液型を Well,/ you re very serious person/ so/ I think/ your blood type is A. えーと / あなたはとっても真面目な人 / だから / 私は ~ と思います / あなたの血液型は
L1 What Can You Blood Type Tell Us? Part 1 Can you guess/ my blood type? 当ててみて / 私の血液型を Well,/ you re very serious person/ so/ I think/ your blood type is A. えーと / あなたはとっても真面目な人 / だから / 私は ~ と思います / あなたの血液型は
 VOL. 17 NO. 7 CHEMOTHERAPY 1305 1) W. BRumFirr et al. : Clinical and laboratory studies with carbenicillin. Lancet 1: 1289~ 1293, 1967 2) E. T. KNUDSEN et al. : A new semisynthetic penicillin active against
VOL. 17 NO. 7 CHEMOTHERAPY 1305 1) W. BRumFirr et al. : Clinical and laboratory studies with carbenicillin. Lancet 1: 1289~ 1293, 1967 2) E. T. KNUDSEN et al. : A new semisynthetic penicillin active against
生命倫理100_資料4-7
 26 27 Single cell clones picked from swaps12p36 (18) Single cell clones picked from swaps12p18 (24) 1.3% (p0) 1.1% (p58) 28 29 Outline 30 31 32 33 34 Columbia, The New York Stem Cell Foundation Oocyte
26 27 Single cell clones picked from swaps12p36 (18) Single cell clones picked from swaps12p18 (24) 1.3% (p0) 1.1% (p58) 28 29 Outline 30 31 32 33 34 Columbia, The New York Stem Cell Foundation Oocyte

 測定した また Scrは酵素法にて測定し その参考基 r =0.575 p
測定した また Scrは酵素法にて測定し その参考基 r =0.575 p