untitled
|
|
|
- たつぞう みおか
- 7 years ago
- Views:
Transcription
1 100
2
3 TNF α TNFα IgG1 TNFα TNFα TNFα Infusion reaction NSAIDs TNFα NSAIDs TNFα Assessment in Ankylosing Spondylitis ASAS International Working Group/European League Against Rheumatism ASAS/EULAR TNFα TNFα 12 NSAIDs TNFα
4 (1) SNSA SNSA ) % ,767,994 8,300 51, , , , , , ) HLA-B27 8 HLA-B27 1% HLA-B27 80%9 HLA-B27 HLA-B TNFα TNFα 12 2
5 1.5 3) Modified New York Criteria TNF ASAS/EULAR X SNSA Amor 1920 Modified New York Criteria 1984 a) 3 b) c) 0 X X 2 3 a) 3 b) X 2 3 4) (a) 10 Braun 1 2a 2b 3 [21] 3
6 1.5 (b) % 20 40% 10 20% 7% 6% 9% 1 4% (2) ASAS/EULAR 1 ASAS/EULAR NSAIDs TNF4
7 1.5 TNF NSAIDs COX NSAIDs 2426 MTX TNF NSAIDs TNF NSAIDs TNFα NSAIDs (1) 30 TNFα NSAIDs 5
8 1.5 TNFα 31 TNFα mrna 32 TNFα 3334 P mg/kg BASDAI 50%5mg/kg 12 5mg/kg 6 48 P mg/kg P P01522 FDA ASSERT 5mg/kg mg/kg ASAS 20% 5mg/kg ASSERT P P01522 ASSERT 5mg/kg 6 P ASSERT 2 P01522 ASSERT 2 6
9 1.5 Remicade is indicated for: Treatment of severe, active ankylosing spondylitis, in adult patients who have responded inadequately to conventional therapy. 5 mg/kg given as an intravenous infusion over a 2-hour period followed by additional 5 mg/kg infusion doses at 2 and 6 weeks after the first infusion, then every 6 to 8 weeks. If a patient does not respond by 6 weeks (i.e. after 2 doses), no additional treatment with infliximab should be given. REMICADE is indicated for reducing signs and symptoms in patients with active ankylosing spondylitis. The recommended dose of REMICADE is 5 mg/kg given as an intravenous infusion followed with additional similar doses at 2 and 6 weeks after the first infusion, then every 6 weeks thereafter. (2) ASSERT 24 ASSERT ASSERT 1 1 ASSERT ASSERT 5mg/kg ASSERT ASAS 20% ASSERT P mg/kg ASAS 20% 24 7
10 1.5 TA mg/kg ASAS 20% 97.0% 32/33 ASSERT
11
12
13 1.5 TA ASSERT C0168T P01522 P mg/kg mg/kg mg/kg 5mg/kg mg/kg 5 mg/kg mg/kg 33 ASAS BASDAI BASMI BASFI CRP SF mg/kg mg/kg 35 ASAS BASDAI BASMI BASFI SF-36 CRP MRI BASDAI BASDAI 2070 BASMI BASFI CRP SF-36 ATI ATI ATI
14 P01522 P ASSERT C0168T51 P01522 ASSERT (1) P01522 P mg/kg A 12 A A mg/kg B P01522 Modified New York Criteria 1984 NSAIDs BASDAI VAS 4 12 BASDAI 50% BASDAI BASFI BASMI SF-36 CRP 5mg/kg 35 A 5mg/kg 5 B BASDAI 50% 5mg/kg 57.1% 20/35 8.6% 3/35 Fisher p<0.015mg/kg BASDAI 50%
15 1.5 5mg/kg (%) * * * BASDAI 50% P mg/kg * Fisher p<0.01 BASDAI BASFI BASMI QOL SF-36 CRP % P mg/kg BASDAI BASFI BASMI (2) ASSERT C0168T mg/kg mg/kg mg/kg 5mg/kg mg/kg 5mg/kg 36 2 BASDAI 3 7.5mg/kg ASSERT 13
16 1.5 Modified New York Criteria 1984 NSAIDs BASDAI VAS 4 24 ASAS 20% AS major clinical response BASDAI BASFI BASMI Chest expansion SF-36 MRI CRP 78 5mg/kg mg/kg 2 24 ASAS 20% 5mg/kg 61.2% 123/ % 15/78 Cochran-Mantel-Haenszel χ 2 p<0.0015mg/kg ASAS 20% ASAS 20ASSERT 24 * Cochran-Mantel-Haenszel χ 2 AS major clinical response BASDAI BASFI BASMI Chest expansionqol SF-36 MRI CRP mg/kg 7 3.5% 5mg/kg Infusion reaction 14
17 mg/kg 5mg/kg 5mg/kg mg/kg 5 or 7.5mg/kg 5 or 7.5mg/kg mg/kg %102 5mg/kg 17 5 or 7.5mg/kg ASAS 20%5mg/kg 62.5% 72.1% 5 or 7.5mg/kg 62.4% 73.9% 5 or 7.5mg/kg 5mg/kg 5mg/kg 83.5% 90.5% 1 7.5mg/kg 7.5mg/kg 44.3% 60.0% ASAS 20ASSERT 102 5mg/kg 5 or 7.5mg/kg a) b) 5mg/kg 7.5mg/kg b) % (9/76) 49.8% (100/201) 67.4% (64/95) 34.0% (36/106) % (14/76) 61.0% (122/200) 77.9% (74/95) 45.7% (48/105) % (15/75) 61.8% (123/199) 81.9% (77/94) 43.8% (46/105) % (18/75) 65.3% (130/199) 86.0% (80/93) 47.2% (50/106) % (14/75) 61.1% (121/198) 81.5% (75/92) 43.4% (46/106) % (45/72) 62.4% (123/197) 83.5% (76/91) 44.3% (47/106) % (44/67) 69.3% (122/176) 90.5% (76/84) 50.0% (46/92) % (41/64) 71.0% (120/169) 90.2% (74/82) 52.9% (46/87) % (44/61) 73.9% (122/165) 88.8% (71/80) 60.0% (51/85) a) mg/kg b) mg/kg 7.5mg/kg % % Infusion reaction 2 0.7% (1) TA mg/kg
18 1.5 Modified New York Criteria 1984 NSAIDs BASDAI VAS 4 24 ASAS 20% AS major clinical response BASDAI BASFI BASMI SF-36 CRP MTX ASAS 20% 97.0% 32/33 ASSERT ASAS 20% % mg/kg ASAS AS major clinical response BASDAI BASFI BASMI QOL SF-36 CRP ASSERT
19 Zochling J, van der Heijde D, Burgos-Vargas R, Collantes E, Davis JC, Jr., Dijkmans B, et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2006;65(4): Keat A, Barkham N, Bhalla A, Gaffney K, Marzo-Ortega H, Paul S, et al. BSR guidelines for prescribing TNF-alpha blockers in adults with ankylosing spondylitis. Report of a working party of the British Society for Rheumatology. Rheumatology (Oxford) 2005;44(7): ;27: Tsujimoto M. Epidemiological research on the prevalence of ankylosing spondylitis. Med J Osaka Univ 1978;28(3-4): Hukuda S, Minami M, Saito T, Mitsui H, Matsui N, Komatsubara Y, et al. Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J Rheumatol 2001;28(3): Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet 1973;1(7809): Khan MA, Khan MK. HLA-B27 as an aid to diagnosis of ankylosing spondylitis. SPINE:State of the Art Reviews 1990;4(3): : : vol.2: van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27(4): Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359(9313): van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52(2): Davis JC, Jr., Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg DO, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003;48(11): van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BA, Braun J, et al. Efficacy and 17
20 1.5 safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;54(7): Amor B, Dougados M, Mijiyawa M. [Criteria of the classification of spondylarthropathies]. Rev Rhum Mal Osteoartic 1990;57(2):85-9.French. 20 Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991;34(10): Sieper J, Appel H, Braun J, Rudwaleit M. Critical appraisal of assessment of structural damage in ankylosing spondylitis: implications for treatment outcomes. Arthritis Rheum 2008;58(3): Medical Practice 2006;23(2): Shaikh SA. Ankylosing spondylitis: recent breakthroughs in diagnosis and treatment. JCCA J Can Chiropr Assoc 2007;51(4): Dougados M, van der Linden S, Leirisalo-Repo M, Huitfeldt B, Juhlin R, Veys E, et al. Sulfasalazine in the treatment of spondylarthropathy. A randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheum 1995;38(5): Clegg DO, Reda DJ, Abdellatif M. Comparison of sulfasalazine and placebo for the treatment of axial and peripheral articular manifestations of the seronegative spondylarthropathies: a Department of Veterans Affairs cooperative study. Arthritis Rheum 1999;42(11): Chen J, liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev 2005(2):CD Gonzalez-Lopez L, Garcia-Gonzalez A, Vazquez-Del-Mercado M, Munoz-Valle JF, Gamez-Nava JI. Efficacy of methotrexate in ankylosing spondylitis: a randomized, double blind, placebo controlled trial. J Rheumatol 2004;31(8): Chen J, Liu C. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev 2004(3):CD Maugars Y, Mathis C, Berthelot JM, Charlier C, Prost A. Assessment of the efficacy of sacroiliac corticosteroid injections in spondylarthropathies: a double-blind study. Br J Rheumatol 1996;35(8): Zink A, Braun J, Listing J, Wollenhaupt J. Disability and handicap in rheumatoid arthritis and ankylosing spondylitis--results from the German rheumatological database. German Collaborative Arthritis Centers. J Rheumatol 2000;27(3): Gratacos J, Collado A, Filella X, Sanmarti R, Canete J, Llena J, et al. Serum cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Br J Rheumatol 1994;33(10): Braun J, Bollow M, Neure L, Seipelt E, Seyrekbasan F, Herbst H, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from 18
21 1.5 patients with ankylosing spondylitis. Arthritis Rheum 1995;38(4): Van den Bosch F, Kruithof E, Baeten D, De Keyser F, Mielants H, Veys EM. Effects of a loading dose regimen of three infusions of chimeric monoclonal antibody to tumour necrosis factor alpha (infliximab) in spondyloarthropathy: an open pilot study. Ann Rheum Dis 2000;59(6): Brandt J, Haibel H, Cornely D, Golder W, Gonzalez J, Reddig J, et al. Successful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor alpha monoclonal antibody infliximab. Arthritis Rheum 2000;43(6): Breban M, Vignon E, Claudepierre P, et al. Efficacy of infliximab in severe refractory ankylosing spondylitis (AS). Results of a 6 months follow-up open-label study. Arthritis & Rheumatism, vol.44, No. 9 supplement, S89, 222, Presented at the First International Workshop on New Treatment Strategies in Ankylosing Spondylitis. Berlin; January 18-19, Kruithof E, Van den Bosch F, Baeten D, Herssens A, De Keyser F, Mielants H, et al. Repeated infusions of infliximab, a chimeric anti-tnfalpha monoclonal antibody, in patients with active spondyloarthropathy: one year follow up. Ann Rheum Dis 2002;61(3): Baraliakos X, Listing J, Fritz C, Alten R, Burmester G, Krause A, et al. Persistent clinical efficacy and safety of infliximab in patients with ankylosing spondylitis over 7 years evidence for different response to anti-tnf-α therapy. Ann Rheum Dis 2008;67:
22 Summary of Product Characteristics Company Core Data Sheet 1
23 () () () ( ) (MTX ) () ( ) ( ) () () ( ) () ( ) () (EU) () () ( ) () () (EU) () () ( )
24 (EU) () () (EU) () () () () (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( )
25 (EU) () () (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( ) () ()
26 (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( )
27 (EU) () () (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( ) () ()
28 (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( ) () () (EU) () () ( )
29 (EU) () () (EU) () () ( ) () () (EU) () () ( ) () () ( ) () () () () () ( ) () ()
30 () () () ( ) () () () () () () () ( ) () ()
31 () () () () () ( ) () () ()
32 () () () () () () ( ) () () () ( ) () () () ()
33 () () () ( ) () () () () () () () () () ()
34 () ( ) () () () () () () ( ) () () () ( ) ()
35 () () () () () () ( ) () () () () () () ( ) () () () () () () ( )
36 () () () () () ( ) () () () ( ) () () () () ()
37 () () () () ( ) ( ) () () () ( ) () () () () () ()
38 () () () () () ( ) () () () ( ) () () ()
39 ( ) () ( ) ()
40 STN: BL /5234 HSTCL PAS April 14, 2009 REMICADE (infliximab) for IV Injection WARNINGS RISK OF SERIOUS INFECTIONS Patients treated with REMICADE are at increased risk for developing serious infections that may lead to hospitalization or death (see WARNINGS and ADVERSE REACTIONS). Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids. REMICADE should be discontinued if a patient develops a serious infection or sepsis. Reported infections include: Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. Patients should be tested for latent tuberculosis before REMICADE use and during therapy. 1,2 Treatment for latent infection should be initiated prior to REMICADE use. Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Empiric anti-fungal therapy should be considered in patients at risk for invasive fungal infections who develop severe systemic illness. Bacterial, viral and other infections due to opportunistic pathogens. The risks and benefits of treatment with REMICADE should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with REMICADE, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy. HEPATOSPLENIC T-CELL LYMPHOMAS Postmarketing cases of hepatosplenic T-cell lymphoma, a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers including REMICADE. These cases have had a very aggressive disease course and have been fatal. All reported REMICADE cases have occurred in patients with Crohn s disease or ulcerative colitis and 1 of 57
41 STN: BL /5234 HSTCL PAS April 14, 2009 the majority were in adolescent and young adult males. All of these patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with REMICADE at or prior to diagnosis. DESCRIPTION REMICADE is a chimeric IgG1κ monoclonal antibody with an approximate molecular weight of 149,100 daltons. It is composed of human constant and murine variable regions. Infliximab binds specifically to human tumor necrosis factor alpha (TNFα) with an association constant of M -1. Infliximab is produced by a recombinant cell line cultured by continuous perfusion and is purified by a series of steps that includes measures to inactivate and remove viruses. REMICADE is supplied as a sterile, white, lyophilized powder for intravenous infusion. Following reconstitution with 10 ml of Sterile Water for Injection, USP, the resulting ph is approximately 7.2. Each single-use vial contains 100 mg infliximab, 500 mg sucrose, 0.5 mg polysorbate 80, 2.2 mg monobasic sodium phosphate, monohydrate, and 6.1 mg dibasic sodium phosphate, dihydrate. No preservatives are present. CLINICAL PHARMACOLOGY General Infliximab neutralizes the biological activity of TNFα by binding with high affinity to the soluble and transmembrane forms of TNFα and inhibits binding of TNFα with its receptors. 3,4 Infliximab does not neutralize TNFβ (lymphotoxin α), a related cytokine that utilizes the same receptors as TNFα. Biological activities attributed to TNFα include: induction of proinflammatory cytokines such as interleukins (IL) 1 and 6, enhancement of leukocyte migration by increasing endothelial layer permeability and expression of adhesion molecules by endothelial cells and leukocytes, activation of neutrophil and eosinophil functional activity, induction of acute phase reactants and other liver proteins, as well as tissue degrading enzymes produced by synoviocytes and/or chondrocytes. Cells expressing transmembrane TNFα bound by infliximab can be lysed in vitro 4 or in vivo. 5 Infliximab inhibits the functional activity of TNFα in a wide variety of in vitro bioassays utilizing human fibroblasts, endothelial cells, neutrophils, B and T lymphocytes and epithelial cells. The relationship of these biological response markers to the mechanism(s) by which REMICADE exerts its clinical effects is unknown. Anti-TNFα antibodies reduce disease activity in the cotton-top tamarin colitis model, and decrease synovitis and joint erosions in a murine model of collagen-induced arthritis. Infliximab prevents disease in transgenic mice that develop polyarthritis as a result of constitutive expression of human TNFα, and when administered after disease onset, allows eroded joints to heal. 2 of 57
42 STN: BL /5234 HSTCL PAS April 14, 2009 Pharmacodynamics Elevated concentrations of TNFα have been found in involved tissues and fluids of patients with rheumatoid arthritis, Crohn s disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis and plaque psoriasis. In rheumatoid arthritis, treatment with REMICADE reduced infiltration of inflammatory cells into inflamed areas of the joint as well as expression of molecules mediating cellular adhesion [E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)], chemoattraction [IL-8 and monocyte chemotactic protein (MCP-1)] and tissue degradation [matrix metalloproteinase (MMP) 1 and 3]. In Crohn s disease, treatment with REMICADE reduced infiltration of inflammatory cells and TNFα production in inflamed areas of the intestine, and reduced the proportion of mononuclear cells from the lamina propria able to express TNFα and interferon. After treatment with REMICADE, patients with rheumatoid arthritis or Crohn s disease exhibited decreased levels of serum IL-6 and C-reactive protein (CRP) compared to baseline. Peripheral blood lymphocytes from REMICADE-treated patients showed no significant decrease in number or in proliferative responses to in vitro mitogenic stimulation when compared to cells from untreated patients. In psoriatic arthritis, treatment with REMICADE resulted in a reduction in the number of T-cells and blood vessels in the synovium and psoriatic skin lesions as well as a reduction of macrophages in the synovium. In plaque psoriasis, REMICADE treatment may reduce the epidermal thickness and infiltration of inflammatory cells. The relationship between these pharmacodynamic activities and the mechanism(s) by which REMICADE exerts its clinical effects is unknown. Pharmacokinetics In adults, single intravenous (IV) infusions of 3 mg/kg to 20 mg/kg showed a linear relationship between the dose administered and the maximum serum concentration. The volume of distribution at steady state was independent of dose and indicated that infliximab was distributed primarily within the vascular compartment. Pharmacokinetic results for single doses of 3 mg/kg to 10 mg/kg in rheumatoid arthritis, 5 mg/kg in Crohn s disease, and 3 mg/kg to 5 mg/kg in plaque psoriasis indicate that the median terminal half-life of infliximab is 7.7 to 9.5 days. Following an initial dose of REMICADE, repeated infusions at 2 and 6 weeks resulted in predictable concentration-time profiles following each treatment. No systemic accumulation of infliximab occurred upon continued repeated treatment with 3 mg/kg or 10 mg/kg at 4- or 8 week intervals. Development of antibodies to infliximab increased infliximab clearance. At 8 weeks after a maintenance dose of 3 to 10 mg/kg of REMICADE, median infliximab serum concentrations ranged from approximately 0.5 to 6 mcg/ml; however, infliximab concentrations were not detectable (<0.1 mcg/ml) in patients who became positive for antibodies to infliximab. No major differences in clearance or volume of distribution were observed in patient subgroups defined by age, weight, or gender. It is not known if there are differences in clearance or volume of distribution in patients with marked impairment of hepatic or renal function. Infliximab peak and trough concentrations were similar in pediatric (aged 6 to 17 years old) and adult patients with Crohn s disease following the administration of the recommended regimen (see DOSAGE AND ADMINISTRATION, Crohn s Disease or Fistulizing Crohn s Disease). 3 of 57
43 STN: BL /5234 HSTCL PAS April 14, 2009 Population pharmacokinetic analysis showed that in children with juvenile rheumatoid arthritis (JRA) with a body weight of up to 35 kg receiving 6 mg/kg REMICADE and children with JRA with body weight greater than 35 kg up to adult body weight receiving 3mg/kg REMICADE, the steady state area under the concentration curve (AUCss) was similar to that observed in adults receiving 3 mg/kg of REMICADE. CLINICAL STUDIES Rheumatoid Arthritis The safety and efficacy of REMICADE were assessed in two multicenter, randomized, doubleblind, pivotal trials: ATTRACT (Study RA I) and ASPIRE (Study RA II). Concurrent use of stable doses of folic acid, oral corticosteroids ( 10 mg/day) and/or non-steroidal antiinflammatory drugs was permitted. Study RA I was a placebo-controlled study of 428 patients with active rheumatoid arthritis despite treatment with MTX. Patients enrolled had a median age of 54 years, median disease duration of 8.4 years, median swollen and tender joint count of 20 and 31 respectively, and were on a median dose of 15 mg/wk of MTX. Patients received either placebo + MTX or one of 4 doses/schedules of REMICADE + MTX: 3 mg/kg or 10 mg/kg of REMICADE by IV infusion at weeks 0, 2 and 6 followed by additional infusions every 4 or 8 weeks in combination with MTX. Study RA II was a placebo-controlled study of three active treatment arms in 1004 MTX naive patients of 3 or fewer years duration active rheumatoid arthritis. Patients enrolled had a median age of 51 years with a median disease duration of 0.6 years, median swollen and tender joint count of 19 and 31, respectively, and >80% of patients had baseline joint erosions. At randomization, all patients received MTX (optimized to 20 mg/wk by week 8) and either placebo, 3mg/kg or 6 mg/kg REMICADE at weeks 0, 2, and 6 and every 8 weeks thereafter. Data on use of REMICADE without concurrent MTX are limited (see ADVERSE REACTIONS, Immunogenicity). 6,7 Clinical response In Study RA I, all doses/schedules of REMICADE + MTX resulted in improvement in signs and symptoms as measured by the American College of Rheumatology response criteria (ACR 20) with a higher percentage of patients achieving an ACR 20, 50 and 70 compared to placebo + MTX (Table 1). This improvement was observed at week 2 and maintained through week 102. Greater effects on each component of the ACR 20 were observed in all patients treated with REMICADE + MTX compared to placebo + MTX (Table 2). More patients treated with REMICADE reached a major clinical response than placebo-treated patients (Table 1). In Study RA II, after 54 weeks of treatment, both doses of REMICADE + MTX resulted in statistically significantly greater response in signs and symptoms compared to MTX alone as measured by the proportion of patients achieving ACR 20, 50 and 70 responses (Table 1). More patients treated with REMICADE reached a major clinical response than placebo-treated patients (Table 1). 4 of 57
44 STN: BL /5234 HSTCL PAS April 14, 2009 Table 1 ACR RESPONSE (PERCENT OF PATIENTS) Study RA I REMICADE + MTX Study RA II REMICADE + MTX 3 mg/kg 10 mg/kg 3 mg/kg 6 mg/kg Placebo q 8 q 4 q 8 q 4 Placebo q 8 q 8 Response + MTX wks wks wks wks + MTX wks wks (n=88) (n=86) (n=86) (n=87) (n=81) (n=274) (n=351) (n=355) ACR 20 Week 30 20% 50% a 50% a 52% a 58% a N/A N/A N/A Week 54 17% 42% a 48% a 59% a 59% a 54% 62% c 66% a ACR 50 Week 30 5% 27% a 29% a 31% a 26% a N/A N/A N/A Week 54 9% 21% c 34% a 40% a 38% a 32% 46% a 50% a ACR 70 Week 30 0% 8% b 11% b 18% a 11% a N/A N/A N/A Week 54 2% 11% c 18% a 26% a 19% a 21% 33% b 37% a Major clinical # response 0% 7% c 8% b 15% a 6% c 8% 12% 17% a # A major clinical response was defined as a 70% ACR response for 6 consecutive months (consecutive visits spanning at least 26 weeks) through week 102 for Study RA I and week 54 for Study RA II. a p b p < 0.01 c p < of 57
45 STN: BL /5234 HSTCL PAS April 14, 2009 Table 2 COMPONENTS OF ACR 20 AT BASELINE AND 54 WEEKS (Study RA I) Placebo + MTX REMICADE + MTX a (n=88) (n=340) Parameter (medians) Baseline Week 54 Baseline Week 54 No. of Tender Joints No. of Swollen Joints Pain b Physician s Global Assessment b Patient s Global Assessment b Disability Index (HAQ DI) c CRP (mg/dl) a All doses/schedules of REMICADE + MTX b Visual Analog Scale (0=best, 10=worst) c Health Assessment Questionnaire, measurement of 8 categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities (0=best, 3=worst) Radiographic response Structural damage in both hands and feet was assessed radiographically at week 54 by the change from baseline in the van der Heijde-modified Sharp (vdh-s) score, a composite score of structural damage that measures the number and size of joint erosions and the degree of joint space narrowing in hands/wrists and feet. 8 In Study RA I, approximately 80% of patients had paired x-ray data at 54 weeks and approximately 70% at 102 weeks. The inhibition of progression of structural damage was observed at 54 weeks (Table 3) and maintained through 102 weeks. In Study RA II, >90% of patients had at least two evaluable x-rays. Inhibition of progression of structural damage was observed at weeks 30 and 54 (Table 3) in the REMICADE + MTX groups compared to MTX alone. Patients treated with REMICADE + MTX demonstrated less progression of structural damage compared to MTX alone, whether baseline acute phase reactants (ESR and CRP) were normal or elevated: patients with elevated baseline acute phase reactants treated with MTX alone demonstrated a mean progression in vdh-s score of 4.2 units compared to patients treated with REMICADE + MTX who demonstrated 0.5 units of progression; patients with normal baseline acute phase reactants treated with MTX alone demonstrated a mean progression in vdh-s score of 1.8 units compared to REMICADE + MTX 6 of 57
46 STN: BL /5234 HSTCL PAS April 14, 2009 who demonstrated 0.2 units of progression. Of patients receiving REMICADE + MTX, 59% had no progression (vdh-s score 0 unit) of structural damage compared to 45% patients receiving MTX alone. In a subset of patients who began the study without erosions, REMICADE + MTX maintained an erosion free state at 1 year in a greater proportion of patients than MTX alone, 79% (77/98) vs. 58% (23/40), respectively (p<0.01). Fewer patients in the REMICADE + MTX groups (47%) developed erosions in uninvolved joints compared to MTX alone (59%). Table 3 RADIOGRAPHIC CHANGE FROM BASELINE TO WEEK 54 Study RA I REMICADE + MTX Study RA II REMICADE + MTX 3 mg/kg 10 mg/kg 3 mg/kg 6 mg/kg Placebo q 8 q 8 Placebo q 8 q 8 + MTX wks wks + MTX wks wks (n=64) (n=71) (n=77) (n=282) (n=359) (n=363) Total Score Baseline Mean Median Change from baseline Mean a 0.2 a a 0.5 a Median Erosion Score Baseline Mean Median Change from baseline Mean a 0.2 a a 0.1 a Median JSN Score Baseline Mean Median Change from baseline Mean a 0.0 a a 0.2 Median a P <0.001 for each outcome against placebo. 7 of 57
47 STN: BL /5234 HSTCL PAS April 14, 2009 Physical function response Physical function and disability were assessed using the Health Assessment Questionnaire (HAQ-DI) and the general health-related quality of life questionnaire SF-36. In Study RA I, all doses/schedules of REMICADE + MTX showed significantly greater improvement from baseline in HAQ-DI and SF-36 physical component summary score averaged over time through week 54 compared to placebo + MTX, and no worsening in the SF-36 mental component summary score. The median (interquartile range) improvement from baseline to week 54 in HAQ-DI was 0.1 (-0.1, 0.5) for the placebo + MTX group and 0.4 (0.1, 0.9) for REMICADE + MTX (p<0.001). Both HAQ-DI and SF-36 effects were maintained through week 102. Approximately 80% of patients in all doses/schedules of REMICADE + MTX remained in the trial through 102 weeks. In Study RA II, both REMICADE treatment groups showed greater improvement in HAQ-DI from baseline averaged over time through week 54 compared to MTX alone; 0.7 for REMICADE + MTX vs. 0.6 for MTX alone (p 0.001). No worsening in the SF-36 mental component summary score was observed. Active Crohn s Disease The safety and efficacy of single and multiple doses of REMICADE were assessed in two randomized, double-blind, placebo-controlled clinical studies in 653 patients with moderate to severely active Crohn s disease [Crohn s Disease Activity Index (CDAI) 220 and 400] with an inadequate response to prior conventional therapies. Concomitant stable doses of aminosalicylates, corticosteroids and/or immunomodulatory agents were permitted and 92% of patients continued to receive at least one of these medications. In the single-dose trial 9 of 108 patients, 16% (4/25) of placebo patients achieved a clinical response (decrease in CDAI 70 points) at week 4 vs. 81% (22/27) of patients receiving 5 mg/kg REMICADE (p<0.001, two-sided, Fisher s Exact test). Additionally, 4% (1/25) of placebo patients and 48% (13/27) of patients receiving 5 mg/kg REMICADE achieved clinical remission (CDAI<150) at week 4. In a multidose trial (ACCENT I [Study Crohn s I]) 10, 545 patients received 5 mg/kg at week 0 and were then randomized to one of three treatment groups; the placebo maintenance group received placebo at weeks 2 and 6, and then every 8 weeks; the 5 mg/kg maintenance group received 5 mg/kg at weeks 2 and 6, and then every 8 weeks; and the 10 mg/kg maintenance group received 5 mg/kg at weeks 2 and 6, and then 10 mg/kg every 8 weeks. Patients in response at week 2 were randomized and analyzed separately from those not in response at week 2. Corticosteroid taper was permitted after week 6. At week 2, 57% (311/545) of patients were in clinical response. At week 30, a significantly greater proportion of these patients in the 5 mg/kg and 10 mg/kg maintenance groups achieved clinical remission compared to patients in the placebo maintenance group (Table 4). 8 of 57
48 STN: BL /5234 HSTCL PAS April 14, 2009 Additionally, a significantly greater proportion of patients in the 5 mg/kg and 10 mg/kg REMICADE maintenance groups were in clinical remission and were able to discontinue corticosteroid use compared to patients in the placebo maintenance group at week 54 (Table 4). Table 4 CLINICAL REMISSION AND STEROID WITHDRAWAL Single 5 mg/kg Dose a Placebo Maintenance Three Dose Induction b REMICADE Maintenance q 8 wks 5 mg/kg 10 mg/kg Week 30 25/102 41/104 48/105 Clinical remission 25% 39% 46% p-value c Week 54 Patients in remission able to 6/54 14/56 18/53 discontinue corticosteroid use d 11% 25% 34% p-value c a REMICADE at week 0 b REMICADE 5 mg/kg administered at weeks 0, 2 and 6 c p-values represent pairwise comparisons to placebo d Of those receiving corticosteroids at baseline Patients in the REMICADE maintenance groups (5 mg/kg and 10 mg/kg) had a longer time to loss of response than patients in the placebo maintenance group (Figure 1). At weeks 30 and 54, significant improvement from baseline was seen among the 5 mg/kg and 10 mg/kg REMICADEtreated groups compared to the placebo group in the disease specific inflammatory bowel disease questionnaire (IBDQ), particularly the bowel and systemic components, and in the physical component summary score of the general health-related quality of life questionnaire SF of 57
49 STN: BL /5234 HSTCL PAS April 14, 2009 Patients Who had Not Lost Response (%) Compared to placebo maintenance: Infliximab 10 mg/kg: p < Infliximab 5 mg/kg: p = Study Week Placebo maintenance 5 mg/kg infliximab maintenance 10 mg/kg infliximab maintenance Figure 1 Kaplan-Meier estimate of the proportion of patients who had not lost response through week 54 In a subset of 78 patients who had mucosal ulceration at baseline and who participated in an endoscopic substudy, 13 of 43 patients in the REMICADE maintenance group had endoscopic evidence of mucosal healing compared to 1 of 28 patients in the placebo group at week 10. Of the REMICADE-treated patients showing mucosal healing at week 10, 9 of 12 patients also showed mucosal healing at week 54. Patients who achieved a response and subsequently lost response were eligible to receive REMICADE on an episodic basis at a dose that was 5 mg/kg higher than the dose to which they were randomized. The majority of such patients responded to the higher dose. Among patients who were not in response at week 2, 59% (92/157) of REMICADE maintenance patients responded by week 14 compared to 51% (39/77) of placebo maintenance patients. Among patients who did not respond by week 14, additional therapy did not result in significantly more responses (see DOSAGE AND ADMINISTRATION). Fistulizing Crohn s Disease The safety and efficacy of REMICADE were assessed in 2 randomized, double-blind, placebocontrolled studies in patients with fistulizing Crohn s disease with fistula(s) that were of at least 3 months duration. Concurrent use of stable doses of corticosteroids, 5-aminosalicylates, antibiotics, MTX, 6-mercaptopurine (6-MP) and/or azathioprine (AZA) was permitted. 10 of 57
50 STN: BL /5234 HSTCL PAS April 14, 2009 In the first trial, patients received three doses of either placebo or REMICADE at weeks 0, 2 and 6. Fistula response ( 50% reduction in number of enterocutaneous fistulas draining upon gentle compression on at least two consecutive visits without an increase in medication or surgery for Crohn s disease) was seen in 68% (21/31) of patients in the 5 mg/kg REMICADE group (p=0.002) and 56% (18/32) of patients in the 10 mg/kg REMICADE group (p=0.021) vs. 26% (8/31) of patients in the placebo arm. The median time to onset of response and median duration of response in REMICADE-treated patients was 2 and 12 weeks, respectively. Closure of all fistula was achieved in 52% of REMICADE-treated patients compared with 13% of placebo-treated patients (p<0.001). In the second trial (ACCENT II [Study Crohn s II]), patients who were enrolled had to have at least one draining enterocutaneous (perianal, abdominal) fistula. All patients received 5 mg/kg REMICADE at weeks 0, 2 and 6. Patients were randomized to placebo or 5 mg/kg REMICADE maintenance at week 14. Patients received maintenance doses at week 14 and then every eight weeks through week 46. Patients who were in fistula response (fistula response was defined the same as in the first trial) at both weeks 10 and 14 were randomized separately from those not in response. The primary endpoint was time from randomization to loss of response among those patients who were in fistula response. Among the randomized patients (273 of the 296 initially enrolled), 87% had perianal fistulas and 14% had abdominal fistulas. Eight percent also had rectovaginal fistulas. Greater than 90% of the patients had received previous immunosuppressive and antibiotic therapy. At week 14, 65% (177/273) of patients were in fistula response. Patients randomized to REMICADE maintenance had a longer time to loss of fistula response compared to the placebo maintenance group (Figure 2). At week 54, 38% (33/87) of REMICADE-treated patients had no draining fistulas compared with 22% (20/90) of placebo-treated patients (p=0.02). Compared to placebo maintenance, patients on REMICADE maintenance had a trend toward fewer hospitalizations. 11 of 57
51 STN: BL /5234 HSTCL PAS April 14, 2009 Patients Who had Not Lost Response (%) Compared to placebo maintenance: Infliximab 5 mg/kg: p = Study Week 6402a Placebo maintenance 5 mg/kg infliximab maintenance Figure 2 Life table estimates of the proportion of patients who had not lost fistula response through week 54 Patients who achieved a fistula response and subsequently lost response were eligible to receive REMICADE maintenance therapy at a dose that was 5 mg/kg higher than the dose to which they were randomized. Of the placebo maintenance patients, 66% (25/38) responded to 5 mg/kg REMICADE, and 57% (12/21) of REMICADE maintenance patients responded to 10 mg/kg. Patients who had not achieved a response by week 14 were unlikely to respond to additional doses of REMICADE. Similar proportions of patients in either group developed new fistulas (17% overall) and similar numbers developed abscesses (15% overall). Active Crohn s Disease in Pediatric Patients The safety and efficacy of REMICADE were assessed in a randomized, open-label study (Study Peds Crohn s) in 112 pediatric patients 6 to 17 years old with moderately to severely active Crohn s disease and an inadequate response to conventional therapies. The median age was 13 years and the median Pediatric Crohn s Disease Activity Index (PCDAI) was 40 (on a scale of 0 to 100). All patients were required to be on a stable dose of 6-mercaptopurine, azathioprine, or methotrexate; 35% were also receiving corticosteroids at baseline. 12 of 57
52 STN: BL /5234 HSTCL PAS April 14, 2009 All patients received induction dosing of 5 mg/kg REMICADE at Weeks 0, 2, and 6. At Week 10, 103 patients were randomized to a maintenance regimen of 5 mg/kg REMICADE given either every 8 weeks or every 12 weeks. At Week 10, 88% of patients were in clinical response (defined as a decrease from baseline in the PCDAI score of 15 points and total PCDAI score of 30 points), and 59% were in clinical remission (defined as PCDAI score of 10 points). The proportion of pediatric patients achieving clinical response at Week 10 compared favorably with the proportion of adults achieving a clinical response in Study Crohn s I. The study definition of clinical response in Study Peds Crohn s was based on the PCDAI score, whereas the CDAI score was used in the adult Study Crohn s I. At both Week 30 and Week 54, the proportion of patients in clinical response was greater in the every 8 week treatment group than in the every 12 week treatment group (73% vs. 47% at Week 30, and 64% vs. 33% at Week 54). At both Week 30 and Week 54, the proportion of patients in clinical remission was also greater in the every 8 week treatment group than in the every 12 week treatment group (60% vs. 35% at Week 30, and 56% vs. 24% at Week 54), (Table 5). For patients in Study Peds Crohn s receiving corticosteroids at baseline, the proportion of patients able to discontinue corticosteroids while in remission at Week 30 was 46% for the every 8 week maintenance group and 33% for the every 12 week maintenance group. At Week 54, the proportion of patients able to discontinue corticosteroids while in remission was 46% for the every 8 week maintenance group and 17% for the every 12 week maintenance group. 13 of 57
53 STN: BL /5234 HSTCL PAS April 14, 2009 Table 5 RESPONSE AND REMISSION IN STUDY PEDS CROHN S 5 mg/kg REMICADE Every 8 Week Every 12 Week Treatment Group Treatment Group Patients randomized Clinical Response 1 Week 30 73%** 47% Week 54 64%** 33% Clinical Remission 2 Week 30 60%* 35% Week 54 56%** 24% 1 Defined as a decrease from baseline in the PCDAI score of 15 points and total score of 30 points. 2 Defined as a PCDAI score of 10 points. * p-value < 0.05 **p-value < of 57
54 STN: BL /5234 HSTCL PAS April 14, 2009 Ankylosing Spondylitis The safety and efficacy of REMICADE were assessed in a randomized, multicenter, doubleblind, placebo-controlled study in 279 patients with active ankylosing spondylitis. Patients were between 18 and 74 years of age, and had ankylosing spondylitis as defined by the modified New York criteria for Ankylosing Spondylitis. 12 Patients were to have had active disease as evidenced by both a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score >4 (possible range 0-10) and spinal pain >4 (on a Visual Analog Scale [VAS] of 0-10). Patients with complete ankylosis of the spine were excluded from study participation, and the use of Disease Modifying Anti-Rheumatic Drugs (DMARDs) and systemic corticosteroids were prohibited. Doses of REMICADE 5 mg/kg or placebo were administered intravenously at Weeks 0, 2, 6, 12 and 18. At 24 weeks, improvement in the signs and symptoms of ankylosing spondylitis, as measured by the proportion of patients achieving a 20% improvement in ASAS response criteria (ASAS 20), was seen in 60% of patients in the REMICADE-treated group vs. 18% of patients in the placebo group (p<0.001). Improvement was observed at week 2 and maintained through week 24 (Figure 3 and Table 6). Figure 3 Proportion of patients achieving ASAS 20 response 15 of 57
untitled
 1988 2000 2002 2004 2006 2008 IFN Lamivudine Adefovir Entecavir 1 Total number 560 Sex (male/female) 424/136 Age (years)* 38 (15-68) Duration of treatment (weeks)* 26 (1-592) Follow-up time (years) 75(05-21
1988 2000 2002 2004 2006 2008 IFN Lamivudine Adefovir Entecavir 1 Total number 560 Sex (male/female) 424/136 Age (years)* 38 (15-68) Duration of treatment (weeks)* 26 (1-592) Follow-up time (years) 75(05-21
 A comparison of abdominal versus vaginal hysterectomy for leiomyoma and adenomyosis Kenji ARAHORI, Hisasi KATAYAMA, Suminori NIOKA Department of Obstetrics and Gnecology, National Maizuru Hospital,Kyoto,
A comparison of abdominal versus vaginal hysterectomy for leiomyoma and adenomyosis Kenji ARAHORI, Hisasi KATAYAMA, Suminori NIOKA Department of Obstetrics and Gnecology, National Maizuru Hospital,Kyoto,
untitled
 1-92009 X 22 RA 2009ACR/EULAR MTX 3 TNF 3 IL-6 1 MTX RA Key words rheumatoid arthritisra 2009RA criteria 2009 biologicstnf anti-tnf drugsremission I rheumatoid arthritisra X 1 1 2 II 629-0392 TEL0771-72-1181FAX0771-72-0326
1-92009 X 22 RA 2009ACR/EULAR MTX 3 TNF 3 IL-6 1 MTX RA Key words rheumatoid arthritisra 2009RA criteria 2009 biologicstnf anti-tnf drugsremission I rheumatoid arthritisra X 1 1 2 II 629-0392 TEL0771-72-1181FAX0771-72-0326
広島県獣医学会雑誌24号.indd
 1 1 Study on the effects of dexamethasone administration in acute coccidiosis accompanied by bloody stools TOMOYASU KUROSE Shoubara Veterinary Clinical Center, P. F. A. M. A. A -1-, Nishihonmachi, Shoubara,
1 1 Study on the effects of dexamethasone administration in acute coccidiosis accompanied by bloody stools TOMOYASU KUROSE Shoubara Veterinary Clinical Center, P. F. A. M. A. A -1-, Nishihonmachi, Shoubara,
988 CHEMOTHERAPY NOV. 1971
 988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
988 CHEMOTHERAPY NOV. 1971 VOL. 19 NO. 8 CHEMOTHERAPY 989 Effect of medium-ph and inoculum size on activity of SB-PC heart infusion agar, mcg/ml Sensitivity distribution of Staphylococci to SB-PC in surgical
日本化学療法学会雑誌第51巻第2号
 piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study
 CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
 ON A FEW INFLUENCES OF THE DENTAL CARIES IN THE ELEMENTARY SCHOOL PUPIL BY Teruko KASAKURA, Naonobu IWAI, Sachio TAKADA Department of Hygiene, Nippon Dental College (Director: Prof. T. Niwa) The relationship
ON A FEW INFLUENCES OF THE DENTAL CARIES IN THE ELEMENTARY SCHOOL PUPIL BY Teruko KASAKURA, Naonobu IWAI, Sachio TAKADA Department of Hygiene, Nippon Dental College (Director: Prof. T. Niwa) The relationship
8 The Bulletin of Meiji University of Integrative Medicine API II 61 ASO X 11 7 X-4 6 X m 5 X-2 4 X 3 9 X 11 7 API 0.84 ASO X 1 1 MR-angio
 7-14 2010 1 1 1 2 1 1 1 2 Fontaine II ASO61 3 API ASO ASO ASO API API KKKKKKKKKK ASO Fontaine II API Received April 14, 2009; Accepted July 16, 2009 I arteriosclerosis obliterans: ASO ASO 50 70 1,2 Fontaine
7-14 2010 1 1 1 2 1 1 1 2 Fontaine II ASO61 3 API ASO ASO ASO API API KKKKKKKKKK ASO Fontaine II API Received April 14, 2009; Accepted July 16, 2009 I arteriosclerosis obliterans: ASO ASO 50 70 1,2 Fontaine
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, Sc
 A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
 Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
Jan THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Table 1. Outline of administering doses, routes and sampling times *: 4 ml/hr/kg Bacillus subtilis
 THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Jan. 1987 Jan. 1987 THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Table 1. Outline of administering doses, routes and sampling times *: 4 ml/hr/kg Bacillus subtilis
THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Jan. 1987 Jan. 1987 THE JAPANESE JOURNAL OF ANTIBIOTICS XL-1 Table 1. Outline of administering doses, routes and sampling times *: 4 ml/hr/kg Bacillus subtilis
Fig. 1 Chemical structure of KW-1070
 Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
 CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
日本消化器外科学会雑誌第29巻第9号
 Table 1 Oligonucleotide primers used for RT-PCR and internal probes used for Southern blot hybridization Cytokine Primer Sequence (5'-3') 5' 3' Internal probe s' 3' Internal probe 5' 3' Internal probe
Table 1 Oligonucleotide primers used for RT-PCR and internal probes used for Southern blot hybridization Cytokine Primer Sequence (5'-3') 5' 3' Internal probe s' 3' Internal probe 5' 3' Internal probe
Key words: Antibodies to Leptospira, Tokyo, Uveitis
 Key words: Antibodies to Leptospira, Tokyo, Uveitis Fig. 1 Distribution of Antibody Titers in Age Decade Fig. 3 Distribution of Antibody Titers in each Strain Fig. 2 Correlation between Antibody Titers
Key words: Antibodies to Leptospira, Tokyo, Uveitis Fig. 1 Distribution of Antibody Titers in Age Decade Fig. 3 Distribution of Antibody Titers in each Strain Fig. 2 Correlation between Antibody Titers
Title 外傷性脊髄損傷患者の泌尿器科学的研究第 3 報 : 上部尿路のレ線学的研究並びに腎機能について Author(s) 伊藤, 順勉 Citation 泌尿器科紀要 (1965), 11(4): Issue Date URL
 Title 外傷性脊髄損傷患者の泌尿器科学的研究第 3 報 : 上部尿路のレ線学的研究並びに腎機能について Author(s) 伊藤, 順勉 Citation 泌尿器科紀要 (1965), 11(4): 278-291 Issue Date 1965-04 URL http://hdl.handle.net/2433/112732 Right Type Departmental Bulletin Paper
Title 外傷性脊髄損傷患者の泌尿器科学的研究第 3 報 : 上部尿路のレ線学的研究並びに腎機能について Author(s) 伊藤, 順勉 Citation 泌尿器科紀要 (1965), 11(4): 278-291 Issue Date 1965-04 URL http://hdl.handle.net/2433/112732 Right Type Departmental Bulletin Paper
 1) University Group Diabetes Program: A study of hypoglycemic agents on vascular complica- in patients with adult-onset tions diabetes. I. Design, methods and baseline results. Diabetes 19 (suppl. 2):
1) University Group Diabetes Program: A study of hypoglycemic agents on vascular complica- in patients with adult-onset tions diabetes. I. Design, methods and baseline results. Diabetes 19 (suppl. 2):
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST
 Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
semen quality or those without WBC in semen. In the patients with azoospermia and normal FSH levels (normogonadotropic azzospermia), the antibody (IgG
 CLINICAL STUDIES OF UROGENITAL INFECTIONS WITH CHLAMYDIA TRACHOMA TIS Report 2. The Epidemiology of Chlamydial Infections in Okayama District in Japan and Detection of Antibodies to Chlamydiae in the Sera
CLINICAL STUDIES OF UROGENITAL INFECTIONS WITH CHLAMYDIA TRACHOMA TIS Report 2. The Epidemiology of Chlamydial Infections in Okayama District in Japan and Detection of Antibodies to Chlamydiae in the Sera
Table 1 Characteristics of the study participants in Imari municipal hospital
 Key words: tuberculosis, booster phenomenon, two-step tuberculin test Table 1 Characteristics of the study participants in Imari municipal hospital Fig. 1 Frequency distributions of size of the first tuberculin
Key words: tuberculosis, booster phenomenon, two-step tuberculin test Table 1 Characteristics of the study participants in Imari municipal hospital Fig. 1 Frequency distributions of size of the first tuberculin
VOL. 36 S-3 CHEMOTHERAPY 437
 VOL. 36 S-3 CHEMOTHERAPY 437 438 CHEMOTHERAPY JULY 1988 Fig. 1 Contractile response of gastrointestinal tract to intravenous administration of saline and EM in interdigestive state in dogs (a) : Saline,
VOL. 36 S-3 CHEMOTHERAPY 437 438 CHEMOTHERAPY JULY 1988 Fig. 1 Contractile response of gastrointestinal tract to intravenous administration of saline and EM in interdigestive state in dogs (a) : Saline,
 Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
日本消化器外科学会雑誌第25巻第11号
 Key words: intestinal nerve plexus, hypoxic perfusion, immunohistochemistry, 5-100 protein Table I (A) Sroo staining reactivity of nerve tissue normal partly mildly declined all mildly declined partly
Key words: intestinal nerve plexus, hypoxic perfusion, immunohistochemistry, 5-100 protein Table I (A) Sroo staining reactivity of nerve tissue normal partly mildly declined all mildly declined partly
ñ{ï 01-65
 191252005.2 19 *1 *2 *3 19562000 45 10 10 Abstract A review of annual change in leading rice varieties for the 45 years between 1956 and 2000 in Japan yielded 10 leading varieties of non-glutinous lowland
191252005.2 19 *1 *2 *3 19562000 45 10 10 Abstract A review of annual change in leading rice varieties for the 45 years between 1956 and 2000 in Japan yielded 10 leading varieties of non-glutinous lowland
 EVALUATION OF NOCTURNAL PENILE TUMESCENCE (NPT) IN THE DIFFERENTIAL DIAGNOSIS OF IMPOTENCE Masaharu Aoki, Yoshiaki Kumamoto, Kazutomi Mohri and Kazunori Ohno Department of Urology, Sapporo Medical College
EVALUATION OF NOCTURNAL PENILE TUMESCENCE (NPT) IN THE DIFFERENTIAL DIAGNOSIS OF IMPOTENCE Masaharu Aoki, Yoshiaki Kumamoto, Kazutomi Mohri and Kazunori Ohno Department of Urology, Sapporo Medical College
日本化学療法学会雑誌第59巻第5号
 Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
_念3)医療2009_夏.indd
 Evaluation of the Social Benefits of the Regional Medical System Based on Land Price Information -A Hedonic Valuation of the Sense of Relief Provided by Health Care Facilities- Takuma Sugahara Ph.D. Abstract
Evaluation of the Social Benefits of the Regional Medical System Based on Land Price Information -A Hedonic Valuation of the Sense of Relief Provided by Health Care Facilities- Takuma Sugahara Ph.D. Abstract
Phase II clinical trials for patients with cancer
 II II I 1 I III 14 20 14 20 14 0.05 14 20 1 10 25 20-80 100-200 95 CI (π B π A ) 1.96 [π A (1 π A )/n A + π B (1 π B )/n B ] π A = probability of response rate by treatment A π B = probability of response
II II I 1 I III 14 20 14 20 14 0.05 14 20 1 10 25 20-80 100-200 95 CI (π B π A ) 1.96 [π A (1 π A )/n A + π B (1 π B )/n B ] π A = probability of response rate by treatment A π B = probability of response
VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity of gram nega
 1066 CHEMOTHERAPY MAR. 1975 Table 1 Sensitivity of standard strains VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity
1066 CHEMOTHERAPY MAR. 1975 Table 1 Sensitivity of standard strains VOL. 23 NO. 3 CHEMOTHERAPY 1067 Table 2 Sensitivity of gram positive cocci isolated from various diagnostic materials Table 3 Sensitivity
Fig. 1 Trends of TB incidence rates for all forms and smear-positive pulmonary TB in Kawasaki City and Japan. Incidence=newly notified cases of all fo
 Kekkaku Vol. 79, No. 1: 17-24, 2004 17 (Received 21 Aug. 2003/Accepted 18 Nov. 2003) Fig. 1 Trends of TB incidence rates for all forms and smear-positive pulmonary TB in Kawasaki City and Japan. Incidence=newly
Kekkaku Vol. 79, No. 1: 17-24, 2004 17 (Received 21 Aug. 2003/Accepted 18 Nov. 2003) Fig. 1 Trends of TB incidence rates for all forms and smear-positive pulmonary TB in Kawasaki City and Japan. Incidence=newly
2 The Bulletin of Meiji University of Integrative Medicine 3, Yamashita 10 11
 1-122013 1 2 1 2 20 2,000 2009 12 1 2 1,362 68.1 2009 1 1 9.5 1 2.2 3.6 0.82.9 1.0 0.2 2 4 3 1 2 4 3 Key words acupuncture and moxibustion Treatment with acupuncture, moxibustion and Anma-Massage-Shiatsu
1-122013 1 2 1 2 20 2,000 2009 12 1 2 1,362 68.1 2009 1 1 9.5 1 2.2 3.6 0.82.9 1.0 0.2 2 4 3 1 2 4 3 Key words acupuncture and moxibustion Treatment with acupuncture, moxibustion and Anma-Massage-Shiatsu
 19 OUR EXPERIENCE IN COMBINED BALNEO AND CHRYSOTHERAPY FOR RHEUMATOID ARTHRITIS by Hidemitsu EZAWA (Director: Prof. Hiroshi MORI NAG A), Department ofinternal Medicine, Institute for Thermal Spring Research,
19 OUR EXPERIENCE IN COMBINED BALNEO AND CHRYSOTHERAPY FOR RHEUMATOID ARTHRITIS by Hidemitsu EZAWA (Director: Prof. Hiroshi MORI NAG A), Department ofinternal Medicine, Institute for Thermal Spring Research,
Title 歯性病巣の関連する皮膚疾患におけるビオチンの効用 Author(s) 高橋, 愼一 ; 川島, 淳子 ; 森本, 光明 ; 山根, 源之 Journal, (): - URL Right Posted at the Inst
 Title 歯性病巣の関連する皮膚疾患におけるビオチンの効用 Author(s) 高橋, 愼一 ; 川島, 淳子 ; 森本, 光明 ; 山根, 源之 Journal, (): - URL http://hdl.handle.net/10130/526 Right Posted at the Institutional Resources for Unique Colle Available from
Title 歯性病巣の関連する皮膚疾患におけるビオチンの効用 Author(s) 高橋, 愼一 ; 川島, 淳子 ; 森本, 光明 ; 山根, 源之 Journal, (): - URL http://hdl.handle.net/10130/526 Right Posted at the Institutional Resources for Unique Colle Available from
Web Stamps 96 KJ Stamps Web Vol 8, No 1, 2004
 The Journal of the Japan Academy of Nursing Administration and Policies Vol 8, No 1, pp 43 _ 57, 2004 The Literature Review of the Japanese Nurses Job Satisfaction Research Which the Stamps-Ozaki Scale
The Journal of the Japan Academy of Nursing Administration and Policies Vol 8, No 1, pp 43 _ 57, 2004 The Literature Review of the Japanese Nurses Job Satisfaction Research Which the Stamps-Ozaki Scale
Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII (45)
 Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2305(45) 2306(46) THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 Dec. Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2307(47) 2308(48) THE JAPANESE
Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2305(45) 2306(46) THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 Dec. Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2307(47) 2308(48) THE JAPANESE
 Hirosaki University Repository 妊 娠 可 能 てんかん 女 性 の 治 療 管 理 基 準 設 定 に 関 する 研 Title 究 Author(s) 兼 子, 直 Citation てんかん 治 療 研 究 振 興 財 団 研 究 年 報. 4, 1992, p.24-35 Issue Date 1992 URL http://hdl.handle.net/10129/1905
Hirosaki University Repository 妊 娠 可 能 てんかん 女 性 の 治 療 管 理 基 準 設 定 に 関 する 研 Title 究 Author(s) 兼 子, 直 Citation てんかん 治 療 研 究 振 興 財 団 研 究 年 報. 4, 1992, p.24-35 Issue Date 1992 URL http://hdl.handle.net/10129/1905
Physical and Psychological Effects of Stressors in Female College Students Reizou Mita*1, Konosuke Tomabechi*1, Isao Yamaguchi*1, Naoko Soeno*1, Shuhe
 Physical and Psychological Effects of Stressors in Female College Students Reizou Mita*1, Konosuke Tomabechi*1, Isao Yamaguchi*1, Naoko Soeno*1, Shuhei Kobayashi*2, Mamoru Nishimuta*2, Michiyuki Shimizu*3,
Physical and Psychological Effects of Stressors in Female College Students Reizou Mita*1, Konosuke Tomabechi*1, Isao Yamaguchi*1, Naoko Soeno*1, Shuhei Kobayashi*2, Mamoru Nishimuta*2, Michiyuki Shimizu*3,
<95DB8C9288E397C389C88A E696E6462>
 2011 Vol.60 No.4 p.332 338 Usefulness of regional education program for dietary salt reduction: Self-monitoring of urinary salt excretion Kenichiro YASUTAKE[1] Kayoko SAWANO[1] Shoko YAMAGUCHI[1] Hiroko
2011 Vol.60 No.4 p.332 338 Usefulness of regional education program for dietary salt reduction: Self-monitoring of urinary salt excretion Kenichiro YASUTAKE[1] Kayoko SAWANO[1] Shoko YAMAGUCHI[1] Hiroko
CHEMOTHERAPY APR Fig. 2 The inactivation of aminoglycoside antibiotics by PC-904 Fig. 3 Serum concentration of PC-904 (1) Fig. 4 Urinary recover
 VOL.26 S-2 CHEMOTHERAPY Gentamicin (GM), Dibekacin (DKB), Tobramycin Fig. 1 Protein concentration and protein binding rate Table 2 Protein binding rate of PC-904 in serum of healthy adults, and patients
VOL.26 S-2 CHEMOTHERAPY Gentamicin (GM), Dibekacin (DKB), Tobramycin Fig. 1 Protein concentration and protein binding rate Table 2 Protein binding rate of PC-904 in serum of healthy adults, and patients
1.5 起原又は発見の経緯及び開発の経緯 レミケード R 点滴静注用 100 製造販売承認事項一部変更承認申請書添付資料第 1 部 ( モジュール 1) 1.5 起原又は発見の経緯及び開発の経緯 田辺三菱製薬株式会社 1
 レミケード点滴静注用 100 に関する資料 本資料に記載された情報に係る権利及び内容の責任は田辺 三菱製薬株式会社に帰属するものであり, 当該情報を適正使 用以外の営利目的に利用することはできません. 田辺三菱製薬株式会社 1.5 起原又は発見の経緯及び開発の経緯 レミケード R 点滴静注用 100 製造販売承認事項一部変更承認申請書添付資料第 1 部 ( モジュール 1) 1.5 起原又は発見の経緯及び開発の経緯
レミケード点滴静注用 100 に関する資料 本資料に記載された情報に係る権利及び内容の責任は田辺 三菱製薬株式会社に帰属するものであり, 当該情報を適正使 用以外の営利目的に利用することはできません. 田辺三菱製薬株式会社 1.5 起原又は発見の経緯及び開発の経緯 レミケード R 点滴静注用 100 製造販売承認事項一部変更承認申請書添付資料第 1 部 ( モジュール 1) 1.5 起原又は発見の経緯及び開発の経緯
明海大学歯学雑誌 37‐2/1.秦泉寺
 J Meikai Dent Med 37, 153 158, 8 153 1 7 5ml /1 min Wong-Baker Face Rating Scale FS 5 1 9. g 3 5ml /1 min, FS 1 66 6ml /1 min FS 5 1 9. g 3 6ml /1 min, FS 3 Two Cases of Xerostomia that Showed an Improvement
J Meikai Dent Med 37, 153 158, 8 153 1 7 5ml /1 min Wong-Baker Face Rating Scale FS 5 1 9. g 3 5ml /1 min, FS 1 66 6ml /1 min FS 5 1 9. g 3 6ml /1 min, FS 3 Two Cases of Xerostomia that Showed an Improvement
Studies of Foot Form for Footwear Design (Part 9) : Characteristics of the Foot Form of Young and Elder Women Based on their Sizes of Ball Joint Girth
 Studies of Foot Form for Footwear Design (Part 9) : Characteristics of the Foot Form of Young and Elder Women Based on their Sizes of Ball Joint Girth and Foot Breadth Akiko Yamamoto Fukuoka Women's University,
Studies of Foot Form for Footwear Design (Part 9) : Characteristics of the Foot Form of Young and Elder Women Based on their Sizes of Ball Joint Girth and Foot Breadth Akiko Yamamoto Fukuoka Women's University,
Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution of female patients with blad
 Key words: Female with bladder irritability, Subjective symptoms, Pyuria, Bacteriuria Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution
Key words: Female with bladder irritability, Subjective symptoms, Pyuria, Bacteriuria Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution
 Table 1 Survival rates of infected mice given antibiotic doses producing peak serum a) S. aurcus Smith Challenge dose :7 ~10 (5% mucin) CFU/mouse. LD50: 1 ~103 (5% mucin) CFU/mouse. Table 2 Survival rates
Table 1 Survival rates of infected mice given antibiotic doses producing peak serum a) S. aurcus Smith Challenge dose :7 ~10 (5% mucin) CFU/mouse. LD50: 1 ~103 (5% mucin) CFU/mouse. Table 2 Survival rates
1272 CHEMOTHERAPY MAR. 1975
 1272 CHEMOTHERAPY MAR. 1975 VOL. 23 NO. 3 CHEMOTHERAPY 1273 Fig. 2 Minimal inhibitory concentration of aminoglycosides against 50 strains of Klebsiella Fig. 1 Minimal inhibitory concentration of aminoglycosides
1272 CHEMOTHERAPY MAR. 1975 VOL. 23 NO. 3 CHEMOTHERAPY 1273 Fig. 2 Minimal inhibitory concentration of aminoglycosides against 50 strains of Klebsiella Fig. 1 Minimal inhibitory concentration of aminoglycosides
Experimental and Clinical Studies of Pregnant Hypertension Takashi SHIMAZU Department of Obstetrics and Gynecology, Osaka City University Medical Scho
 Experimental and Clinical Studies of Pregnant Hypertension Takashi SHIMAZU Department of Obstetrics and Gynecology, Osaka City University Medical School While the problem of late pregnant hypertension
Experimental and Clinical Studies of Pregnant Hypertension Takashi SHIMAZU Department of Obstetrics and Gynecology, Osaka City University Medical School While the problem of late pregnant hypertension
Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date URL
 Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date 1958-01 URL http://hdl.handle.net/2433/111559 Right Type Departmental Bulletin
Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date 1958-01 URL http://hdl.handle.net/2433/111559 Right Type Departmental Bulletin
 Effects of the testosterone on both male and female fowls Shohyoe Tsuda Résumé This study was carried out to test the biological actions of androgen on both male and female fowls. As androgen "Amolisin"
Effects of the testosterone on both male and female fowls Shohyoe Tsuda Résumé This study was carried out to test the biological actions of androgen on both male and female fowls. As androgen "Amolisin"
VOL.35 S-2 CHEMOTHERAPY Table 1 Sex and age distribution Table 2 Applications of treatment with carumonam Table 3 Concentration of carumonam in human
 CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
 1) Garner J. S.: The Hospital Infection Control Practices Advisory Committee, CDC, Guidelines for Isolation Precautions in Hospitals, 1996. http://wonder.cdc.gov/wonder/prevguid/ p0000419/p0000419.asp.
1) Garner J. S.: The Hospital Infection Control Practices Advisory Committee, CDC, Guidelines for Isolation Precautions in Hospitals, 1996. http://wonder.cdc.gov/wonder/prevguid/ p0000419/p0000419.asp.
熊本大学学術リポジトリ Kumamoto University Repositor Title 炎症性腸疾患に対する HSF1 及び HSP70 の保護的役割 Author(s) 田中, 健一郎 Citation Issue date Type URL Thesis or Di
 熊本大学学術リポジトリ Kumamoto University Repositor Title 炎症性腸疾患に対する HSF1 及び HSP70 の保護的役割 Author(s) 田中, 健一郎 Citation Issue date 2008-03-25 Type URL Thesis or Dissertation http://hdl.handle.net/2298/9448 Right 1
熊本大学学術リポジトリ Kumamoto University Repositor Title 炎症性腸疾患に対する HSF1 及び HSP70 の保護的役割 Author(s) 田中, 健一郎 Citation Issue date 2008-03-25 Type URL Thesis or Dissertation http://hdl.handle.net/2298/9448 Right 1
女子短大生に対する栄養マネジメント教育とその評価
 10 11 12 http://www.center.ibk.ed.jp/contents/kenkyuu /houkoku/data/030s/sport1.htm http://www.mhlw.go.jp/bunya/kenkou/eiy ou/dl/h24-houkoku.pdf http://www.mhlw.go.jp/bunya/kenkou/dl/ kenkounippon21_02.pdf
10 11 12 http://www.center.ibk.ed.jp/contents/kenkyuu /houkoku/data/030s/sport1.htm http://www.mhlw.go.jp/bunya/kenkou/eiy ou/dl/h24-houkoku.pdf http://www.mhlw.go.jp/bunya/kenkou/dl/ kenkounippon21_02.pdf
 rectomy as the subjects of study, urinary steroid hormones were measured, and simultaneously the enzyme work and enzyme pattern related to the steroid metabolism, as proposed by Yoshida of our laboratory,
rectomy as the subjects of study, urinary steroid hormones were measured, and simultaneously the enzyme work and enzyme pattern related to the steroid metabolism, as proposed by Yoshida of our laboratory,
udc-2.dvi
 13 0.5 2 0.5 2 1 15 2001 16 2009 12 18 14 No.39, 2010 8 2009b 2009a Web Web Q&A 2006 2007a20082009 2007b200720082009 20072008 2009 2009 15 1 2 2 2.1 18 21 1 4 2 3 1(a) 1(b) 1(c) 1(d) 1) 18 16 17 21 10
13 0.5 2 0.5 2 1 15 2001 16 2009 12 18 14 No.39, 2010 8 2009b 2009a Web Web Q&A 2006 2007a20082009 2007b200720082009 20072008 2009 2009 15 1 2 2 2.1 18 21 1 4 2 3 1(a) 1(b) 1(c) 1(d) 1) 18 16 17 21 10
VOL.39 S-3
 VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
2 10 The Bulletin of Meiji University of Integrative Medicine 1,2 II 1 Web PubMed elbow pain baseball elbow little leaguer s elbow acupun
 10 1-14 2014 1 2 3 4 2 1 2 3 4 Web PubMed elbow pain baseball elbow little leaguer s elbow acupuncture electric acupuncture 2003 2012 10 39 32 Web PubMed Key words growth stage elbow pain baseball elbow
10 1-14 2014 1 2 3 4 2 1 2 3 4 Web PubMed elbow pain baseball elbow little leaguer s elbow acupuncture electric acupuncture 2003 2012 10 39 32 Web PubMed Key words growth stage elbow pain baseball elbow
2 94
 32 2008 pp. 93 106 1 Received October 30, 2008 The purpose of this study is to examine the effects of aerobics training class on weight loss for female students in HOKURIKU UNIVERSITY. Seventy four female
32 2008 pp. 93 106 1 Received October 30, 2008 The purpose of this study is to examine the effects of aerobics training class on weight loss for female students in HOKURIKU UNIVERSITY. Seventy four female
 VOL.32 S-9 CHEMOTHERAPY Table 1 Minimum inhibitory concentrations of AC-1370, CPZ and CAZ Table 2 Efficacy of AC-1370 and CPZ against systemic infections in mice *Inoculum size: 106 cells/ml * 95% confidence
VOL.32 S-9 CHEMOTHERAPY Table 1 Minimum inhibitory concentrations of AC-1370, CPZ and CAZ Table 2 Efficacy of AC-1370 and CPZ against systemic infections in mice *Inoculum size: 106 cells/ml * 95% confidence
36:378 第 38 回日本脳卒中学会講演シンポジウム 原著 36: , 要旨 TIA 2 t-pa Key words: stroke registry, stroke subtype, onset-visi
 36:378 第 38 回日本脳卒中学会講演シンポジウム 原著 36: 378 384, 2014 1 2 要旨 1999 2012 10 31 29 26 80 30 TIA 2 t-pa Key words: stroke registry, stroke subtype, onset-visit time, chronological change はじめに 4 12 23 27 1 Japan
36:378 第 38 回日本脳卒中学会講演シンポジウム 原著 36: 378 384, 2014 1 2 要旨 1999 2012 10 31 29 26 80 30 TIA 2 t-pa Key words: stroke registry, stroke subtype, onset-visit time, chronological change はじめに 4 12 23 27 1 Japan
208 ( 2 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 63 _ 3 June 2010 Cefditoren pivoxil (CDTR-PI) MS MS 10%
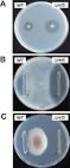 207 ( 1 ) Cefditoren pivoxil G 2010 2 2 2006 3 Cefditoren pivoxil CDTR-PI MS 10% CDTR-PI 305 2,144 2,006 1,958 1.79% 36 2,006 26 (1.30%) CDTR-PI CDTR-PI (9 mg/kg/day) 1.5 2 (2.70%) 2 (1.92%) 93.5% 1,831
207 ( 1 ) Cefditoren pivoxil G 2010 2 2 2006 3 Cefditoren pivoxil CDTR-PI MS 10% CDTR-PI 305 2,144 2,006 1,958 1.79% 36 2,006 26 (1.30%) CDTR-PI CDTR-PI (9 mg/kg/day) 1.5 2 (2.70%) 2 (1.92%) 93.5% 1,831
Fig. 1 Chemical structure of DL-8280
 Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
Fig. 1 Chemical structure of DL-8280 Fig. 2 Susceptibility of cl in ical isolates to DL4280 Fig. 5 Susceptibility of clinical isolates to DL-8280 Fig. 3 Susceptibility of clinical isolates to DL-8280 Fig.
 1) Topley & Wilsons: Principles of Bacterio- 583-591.-5) Huddleson, I.F.: Brucellosis in man and animals, 1943. -6) Wilson, G. S. & encountered in certain Br. abortus agglutinating sera, J. Path. & Bact.,
1) Topley & Wilsons: Principles of Bacterio- 583-591.-5) Huddleson, I.F.: Brucellosis in man and animals, 1943. -6) Wilson, G. S. & encountered in certain Br. abortus agglutinating sera, J. Path. & Bact.,
 DISTRIBUTION OF NEUROPEPTIDES IN THE INFERIOR NASAL TURBINATE MUCOSA OF PATIENTS WITH ALLERGIC RHINITIS KAZUHIRO YAMAMOTO. M.D. Department of Otolaryngology, School of Medicine, Kitasato University, Sagamihara
DISTRIBUTION OF NEUROPEPTIDES IN THE INFERIOR NASAL TURBINATE MUCOSA OF PATIENTS WITH ALLERGIC RHINITIS KAZUHIRO YAMAMOTO. M.D. Department of Otolaryngology, School of Medicine, Kitasato University, Sagamihara
生命倫理100_資料4-7
 26 27 Single cell clones picked from swaps12p36 (18) Single cell clones picked from swaps12p18 (24) 1.3% (p0) 1.1% (p58) 28 29 Outline 30 31 32 33 34 Columbia, The New York Stem Cell Foundation Oocyte
26 27 Single cell clones picked from swaps12p36 (18) Single cell clones picked from swaps12p18 (24) 1.3% (p0) 1.1% (p58) 28 29 Outline 30 31 32 33 34 Columbia, The New York Stem Cell Foundation Oocyte
日赤 No.35☆/7.河相
 2 64 2 Mucosa-Associated Lymphoid TissueMALT T γ- 1 Key words T 1 2 64 47 54 60 MALT 63 T MALT 6062-8 63 1 T MALT 63 CHOP 2 35 28 30 28 75 μg 200 mg 20 mg 200 mg 400 mg/80 mg 2 35 mg 1 2 150 mg 200 mg
2 64 2 Mucosa-Associated Lymphoid TissueMALT T γ- 1 Key words T 1 2 64 47 54 60 MALT 63 T MALT 6062-8 63 1 T MALT 63 CHOP 2 35 28 30 28 75 μg 200 mg 20 mg 200 mg 400 mg/80 mg 2 35 mg 1 2 150 mg 200 mg
indd
 12 61 1 2011 原著 1)2)5) 1) 1)2) 1)2)5) 1)3)5) 1)2) 1)2)5) 1)2) 1)2) 2) 2) 4) 1) 1) 2) 3) 4) 5) 2010 10 6 2010 12 6 A Comparative Study of the Effect and Discomfort Produced by Pharyngeal Anesthesia with
12 61 1 2011 原著 1)2)5) 1) 1)2) 1)2)5) 1)3)5) 1)2) 1)2)5) 1)2) 1)2) 2) 2) 4) 1) 1) 2) 3) 4) 5) 2010 10 6 2010 12 6 A Comparative Study of the Effect and Discomfort Produced by Pharyngeal Anesthesia with
Table 1. Numbers of Cancerous Pleurisy Cases According to Primary Site Table 2. Histological Type of Lung Carcinoma with Cancerous Pleurisy at Autopsy
 Clinicopathological Studies on Cancerous Pleurisy, with Emphasis on Extra-Pulmonary Malignancy Table 1. Numbers of Cancerous Pleurisy Cases According to Primary Site Table 2. Histological Type of Lung
Clinicopathological Studies on Cancerous Pleurisy, with Emphasis on Extra-Pulmonary Malignancy Table 1. Numbers of Cancerous Pleurisy Cases According to Primary Site Table 2. Histological Type of Lung
Mikio Yamamoto: Dynamical Measurement of the E-effect in Iron-Cobalt Alloys. The AE-effect (change in Young's modulus of elasticity with magnetization
 Mikio Yamamoto: Dynamical Measurement of the E-effect in Iron-Cobalt Alloys. The AE-effect (change in Young's modulus of elasticity with magnetization) in the annealed state of iron-cobalt alloys has been
Mikio Yamamoto: Dynamical Measurement of the E-effect in Iron-Cobalt Alloys. The AE-effect (change in Young's modulus of elasticity with magnetization) in the annealed state of iron-cobalt alloys has been
Visual Evaluation of Polka-dot Patterns Yoojin LEE and Nobuko NARUSE * Granduate School of Bunka Women's University, and * Faculty of Fashion Science,
 Visual Evaluation of Polka-dot Patterns Yoojin LEE and Nobuko NARUSE * Granduate School of Bunka Women's University, and * Faculty of Fashion Science, Bunka Women's University, Shibuya-ku, Tokyo 151-8523
Visual Evaluation of Polka-dot Patterns Yoojin LEE and Nobuko NARUSE * Granduate School of Bunka Women's University, and * Faculty of Fashion Science, Bunka Women's University, Shibuya-ku, Tokyo 151-8523
硫酸アルミニウムカリウムタンニン酸注射液(ALTA)による内痔核硬化療法後にフルニエ症候群をきたした1例 第65巻02号0075頁
 65 V A Case Report of Fournier s Gangrene after Sclerosing Therapy for Internal Hemorrhoids with Aluminum Potassium Sulfate and Tannic Acid (ALTA) Injection Aluminum potassium sulfate and tannic acid
65 V A Case Report of Fournier s Gangrene after Sclerosing Therapy for Internal Hemorrhoids with Aluminum Potassium Sulfate and Tannic Acid (ALTA) Injection Aluminum potassium sulfate and tannic acid
STUDIES ON THE RELATION BETWEEN LATE DUMPING SYNDROME AND GLUCAGON RESPONCES TO GLUCOSE Taisuke MATSUI The First Department of Surgery, Nara Medical U
 STUDIES ON THE RELATION BETWEEN LATE DUMPING SYNDROME AND GLUCAGON RESPONCES TO GLUCOSE Taisuke MATSUI The First Department of Surgery, Nara Medical University, Kashihara (Director: Prof. H. Nakano) This
STUDIES ON THE RELATION BETWEEN LATE DUMPING SYNDROME AND GLUCAGON RESPONCES TO GLUCOSE Taisuke MATSUI The First Department of Surgery, Nara Medical University, Kashihara (Director: Prof. H. Nakano) This
CHEMOTHERAPY APR. 1984
 VOL.32 S-3 CHEMOTHERAPY dihydro-4-oxo-7-(1-piperazinyl)-1, 8-naphthyridine- CHEMOTHERAPY APR. 1984 VOL.32 S-3 CHEMOTHERAPY Table 1 Implantation rates and post- implantation survival rates in females mated
VOL.32 S-3 CHEMOTHERAPY dihydro-4-oxo-7-(1-piperazinyl)-1, 8-naphthyridine- CHEMOTHERAPY APR. 1984 VOL.32 S-3 CHEMOTHERAPY Table 1 Implantation rates and post- implantation survival rates in females mated
Kekkaku Vol. 59, No8 STUDIES ON THE ORIGIN AND CLINICAL SIGNIFICANCE OF SMEAR-POSITIVE AND CULTURE-NEGATIVE TUBERCLE BACILLI Michio TSUKAMURA* and Har
 Kekkaku Vol. 59, No8 STUDIES ON THE ORIGIN AND CLINICAL SIGNIFICANCE OF SMEAR-POSITIVE AND CULTURE-NEGATIVE TUBERCLE BACILLI Michio TSUKAMURA* and Haruo TOYAMA (Received for publication April 4, 1984)
Kekkaku Vol. 59, No8 STUDIES ON THE ORIGIN AND CLINICAL SIGNIFICANCE OF SMEAR-POSITIVE AND CULTURE-NEGATIVE TUBERCLE BACILLI Michio TSUKAMURA* and Haruo TOYAMA (Received for publication April 4, 1984)
 Title 生活年令による学級の等質化に関する研究 (1) - 生活年令と学業成績について - Author(s) 与那嶺, 松助 ; 東江, 康治 Citation 研究集録 (5): 33-47 Issue Date 1961-12 URL http://hdl.handle.net/20.500.12000/ Rights 46 STUDIES ON HOMOGENEOUS
Title 生活年令による学級の等質化に関する研究 (1) - 生活年令と学業成績について - Author(s) 与那嶺, 松助 ; 東江, 康治 Citation 研究集録 (5): 33-47 Issue Date 1961-12 URL http://hdl.handle.net/20.500.12000/ Rights 46 STUDIES ON HOMOGENEOUS
こんにちは由美子です
 Sample size power calculation Sample Size Estimation AZTPIAIDS AIDSAZT AIDSPI AIDSRNA AZTPr (S A ) = π A, PIPr (S B ) = π B AIDS (sampling)(inference) π A, π B π A - π B = 0.20 PI 20 20AZT, PI 10 6 8 HIV-RNA
Sample size power calculation Sample Size Estimation AZTPIAIDS AIDSAZT AIDSPI AIDSRNA AZTPr (S A ) = π A, PIPr (S B ) = π B AIDS (sampling)(inference) π A, π B π A - π B = 0.20 PI 20 20AZT, PI 10 6 8 HIV-RNA
 1) i) Barber, M. et al.: Brit. Med J, 2, 565, 19'49. ii) Barber, M.F.G. J. Hayhoe and J. E. M. Whithead: Lancet, 1120 `1125, 1949.-2) Bergey: Bergey's Manual of Determinative Bacteriology 7 th Ed: (1958).-3)
1) i) Barber, M. et al.: Brit. Med J, 2, 565, 19'49. ii) Barber, M.F.G. J. Hayhoe and J. E. M. Whithead: Lancet, 1120 `1125, 1949.-2) Bergey: Bergey's Manual of Determinative Bacteriology 7 th Ed: (1958).-3)
™…
 Review The Secret to Healthy Long Life Decrease in Oxidative and Mental Stress My motto is Health is not all. But nothing can be done without health. Health is the most important requisite for all human
Review The Secret to Healthy Long Life Decrease in Oxidative and Mental Stress My motto is Health is not all. But nothing can be done without health. Health is the most important requisite for all human
CHEMOTHERAPY Fig. 1 Chemical structure of CXM-AX
 Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
CHEMOTHERAPY FEB Table 1 Background of volunteers
 CHEMOTHERAPY FEB. 1985 Table 1 Background of volunteers Table 3 Reproducibility of saisomicln In the EIA and the RIA Fig.1 Comparison of the EIA with the RIA or bioassay of sisomicin Table 4 Blood levels
CHEMOTHERAPY FEB. 1985 Table 1 Background of volunteers Table 3 Reproducibility of saisomicln In the EIA and the RIA Fig.1 Comparison of the EIA with the RIA or bioassay of sisomicin Table 4 Blood levels
 Background : There have not been any informations available of whether diuretics may enhance renoprotective effects of ACEI and ARB. Methods Post-hoc analysis of the results of COOPERATE trial, asking
Background : There have not been any informations available of whether diuretics may enhance renoprotective effects of ACEI and ARB. Methods Post-hoc analysis of the results of COOPERATE trial, asking
 b) Gram-negative bacteria Fig. 2 Sensitivity distribution of clinical isolates : E. coli Fig. 3 Sensitivity distribution of clinical isolates : Pseudomonas Fig. 1 Sensitivity distribution of clinical isolates
b) Gram-negative bacteria Fig. 2 Sensitivity distribution of clinical isolates : E. coli Fig. 3 Sensitivity distribution of clinical isolates : Pseudomonas Fig. 1 Sensitivity distribution of clinical isolates
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates
 Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
過去26年間のスギ花粉飛散パターンのクラスター分析
 117 681 : 2A 2B 2C 2A 2B 2C 2A 2A 2B 2C 2A 2B 2C 2A : DNA Phöbus Blackly 1cm 117 682 2014 1 SYSTAT χ Complete linkage method χ 2A 2B 2C /cm /cm /cm 2A 2B 2C 2A 2B 2C 2A 2B 2C 2 A /cm 2A 2C 117 683 2 2A
117 681 : 2A 2B 2C 2A 2B 2C 2A 2A 2B 2C 2A 2B 2C 2A : DNA Phöbus Blackly 1cm 117 682 2014 1 SYSTAT χ Complete linkage method χ 2A 2B 2C /cm /cm /cm 2A 2B 2C 2A 2B 2C 2A 2B 2C 2 A /cm 2A 2C 117 683 2 2A
Fig. 1 Chemical structure of norfioxacin (AM-715)
 Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
日本化学療法学会雑誌第61巻第4号
 μ μ μ μ μ μ Key words I β μ Sex Age (years) Height (cm) Evaluation items Table1.Characteristics of patients 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) male 12 8 7 27 female 2 1
μ μ μ μ μ μ Key words I β μ Sex Age (years) Height (cm) Evaluation items Table1.Characteristics of patients 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) male 12 8 7 27 female 2 1
...Q.....\1_4.ai
 * *** ** **** * ** *** **** 1910 204203 [] [] [] [] 2004 36 4071822204203 13 t 2 P0.05 [] BMI 68 90% 26.6% 68% 66.2% 37 847% 34% 20.6% 11.3% 34.3% 28.1% 30% 38 31.4%41.9% 145%3 38.7%21.2% B 4 C 35.2% 16.0%
* *** ** **** * ** *** **** 1910 204203 [] [] [] [] 2004 36 4071822204203 13 t 2 P0.05 [] BMI 68 90% 26.6% 68% 66.2% 37 847% 34% 20.6% 11.3% 34.3% 28.1% 30% 38 31.4%41.9% 145%3 38.7%21.2% B 4 C 35.2% 16.0%
 Title 個人 集団レベルの心理社会的学校環境が生体的ストレス反応に及ぼす影響 Author(s) 高倉, 実 ; 小林, 稔 ; 和氣, 則江 ; 安仁屋, 洋子 Citation Issue Date 2007-03 URL http://hdl.handle.net/20.500.12000/ Rights Abstracts of Research Project, Grant-in-Aid
Title 個人 集団レベルの心理社会的学校環境が生体的ストレス反応に及ぼす影響 Author(s) 高倉, 実 ; 小林, 稔 ; 和氣, 則江 ; 安仁屋, 洋子 Citation Issue Date 2007-03 URL http://hdl.handle.net/20.500.12000/ Rights Abstracts of Research Project, Grant-in-Aid
 2) Goetz, A., Tsuneishi, N.: Application of molecular filter membranes to the bacteriological analysis of water, J. Am. Water Works Assn., 43 (12): 943-969,1951. 3) Clark, H.F. et al.: The membrane filter
2) Goetz, A., Tsuneishi, N.: Application of molecular filter membranes to the bacteriological analysis of water, J. Am. Water Works Assn., 43 (12): 943-969,1951. 3) Clark, H.F. et al.: The membrane filter
The Most Effective Exercise for Strengthening the Supraspinatus : Evaluation by Magnetic Resonance Imaging by TAKEDA Yoshitsugu and ENDO Kenji Departm
 The Most Effective Exercise for Strengthening the Supraspinatus : Evaluation by Magnetic Resonance Imaging by TAKEDA Yoshitsugu and ENDO Kenji Department of Orthopedic Surgery, The University of Tokushima
The Most Effective Exercise for Strengthening the Supraspinatus : Evaluation by Magnetic Resonance Imaging by TAKEDA Yoshitsugu and ENDO Kenji Department of Orthopedic Surgery, The University of Tokushima
 VOL. 17 NO. 7 CHEMOTHERAPY 1305 1) W. BRumFirr et al. : Clinical and laboratory studies with carbenicillin. Lancet 1: 1289~ 1293, 1967 2) E. T. KNUDSEN et al. : A new semisynthetic penicillin active against
VOL. 17 NO. 7 CHEMOTHERAPY 1305 1) W. BRumFirr et al. : Clinical and laboratory studies with carbenicillin. Lancet 1: 1289~ 1293, 1967 2) E. T. KNUDSEN et al. : A new semisynthetic penicillin active against
,,.,,.,..,.,,,.,, Aldous,.,,.,,.,,, NPO,,.,,,,,,.,,,,.,,,,..,,,,.,
 J. of Population Problems. pp.,.,,,.,,..,,..,,,,.,.,,...,.,,..,.,,,. ,,.,,.,..,.,,,.,, Aldous,.,,.,,.,,, NPO,,.,,,,,,.,,,,.,,,,..,,,,., ,,.,,..,,.,.,.,,,,,.,.,.,,,. European Labour Force Survey,,.,,,,,,,
J. of Population Problems. pp.,.,,,.,,..,,..,,,,.,.,,...,.,,..,.,,,. ,,.,,.,..,.,,,.,, Aldous,.,,.,,.,,, NPO,,.,,,,,,.,,,,.,,,,..,,,,., ,,.,,..,,.,.,.,,,,,.,.,.,,,. European Labour Force Survey,,.,,,,,,,
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S.
 Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
 Medical Journal of Aizawa Hospital Medical Journal of Aizawa Hospital Vol. 10 (2012) Key words ö γ µ Graves disease and Hashimoto s thyroiditis are autoimmune thyroid diseases. Graves disease causes
Medical Journal of Aizawa Hospital Medical Journal of Aizawa Hospital Vol. 10 (2012) Key words ö γ µ Graves disease and Hashimoto s thyroiditis are autoimmune thyroid diseases. Graves disease causes
VOL. 34 S-2 CHEMOTH8RAPY 913
 VOL. 34 S-2 CHEMOTH8RAPY 913 914 CHEMOTHERAPY APR. 1986 Fig. 1 Chemical structure of T-2588 and T-2525 T- 2588 pivaloyloxymethyl (+ )- (6 R, 7 R)-7-[(Z)-2- (2-amino- 4-thiazolyl)-2-methox yiminoacetamido]-3-[(
VOL. 34 S-2 CHEMOTH8RAPY 913 914 CHEMOTHERAPY APR. 1986 Fig. 1 Chemical structure of T-2588 and T-2525 T- 2588 pivaloyloxymethyl (+ )- (6 R, 7 R)-7-[(Z)-2- (2-amino- 4-thiazolyl)-2-methox yiminoacetamido]-3-[(
CHEMOTHERAPY FEB Table 1. Activity of cefpirome and others against clinical isolates
 VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
VOL.47 NO.5 Table 1. Susceptibility distribution of Ĉ- lactams against clinical isolates of MRSA MRSA: rnethicillin- resistant Staphylococcus aureus
 MAY 1999 VOL.47 NO.5 Table 1. Susceptibility distribution of ƒà- lactams against clinical isolates of MRSA MRSA: rnethicillin- resistant Staphylococcus aureus (oxacillin MIC: 4ƒÊg/ ml) FMOX: flomoxef,
MAY 1999 VOL.47 NO.5 Table 1. Susceptibility distribution of ƒà- lactams against clinical isolates of MRSA MRSA: rnethicillin- resistant Staphylococcus aureus (oxacillin MIC: 4ƒÊg/ ml) FMOX: flomoxef,
CHEMOTHERAPY Fig. 1 Body weight changes of pregnant mice treated orally with AM- 715 Day of sestation
 CHEMOTHERAPY CHEMOTHERAPY Fig. 1 Body weight changes of pregnant mice treated orally with AM- 715 Day of sestation CHEMOTHERAPY Table 1 Preliminaly test of AM- 715 1): Mean } SD *: Significant difference
CHEMOTHERAPY CHEMOTHERAPY Fig. 1 Body weight changes of pregnant mice treated orally with AM- 715 Day of sestation CHEMOTHERAPY Table 1 Preliminaly test of AM- 715 1): Mean } SD *: Significant difference
220 28;29) 30 35) 26;27) % 8.0% 9 36) 8) 14) 37) O O 13 2 E S % % 2 6 1fl 2fl 3fl 3 4
 Vol. 12 No. 2 2002 219 239 Λ1 Λ1 729 1 2 29 4 3 4 5 1) 2) 3) 4 6) 7 27) Λ1 701-0193 288 219 220 28;29) 30 35) 26;27) 0 6 7 12 13 18 59.9% 8.0% 9 36) 8) 14) 37) 1 1 1 13 6 7 O O 13 2 E S 1 1 17 0 6 1 585
Vol. 12 No. 2 2002 219 239 Λ1 Λ1 729 1 2 29 4 3 4 5 1) 2) 3) 4 6) 7 27) Λ1 701-0193 288 219 220 28;29) 30 35) 26;27) 0 6 7 12 13 18 59.9% 8.0% 9 36) 8) 14) 37) 1 1 1 13 6 7 O O 13 2 E S 1 1 17 0 6 1 585
 ;~ (Summary) The Study on the Effects of Foot Bathing on Urination Kumiko Toyoda School of Human Nursing, University of Shiga Prefecture Background Foot bathing is one of the important nursing care for
;~ (Summary) The Study on the Effects of Foot Bathing on Urination Kumiko Toyoda School of Human Nursing, University of Shiga Prefecture Background Foot bathing is one of the important nursing care for
