理想気体ideal gasの熱力学的基本関係式
|
|
|
- こうだい ほがり
- 5 years ago
- Views:
Transcription
1 the equipartition law of energy ( )kt k Boltzmann constant 5 Longman Dictionary of Physics (/)kt q Bq (/)kt equipartition law of energy mol (3/)kT (3/)RT (3/)R (5/)R 3R kt - equipartition of energy The principle of equipartition of energy, based on classical statistical mechanics and enunciated by Boltzmann, states that the mean energy of the molecules of a gas is equally divided among the various degrees of freedom of the molecules. The average energy of each degree of freedom is equal to (/)kt, where k is the Boltzmann constant and T is the thermodynamic temperature. In the late nineteenth century the principle was extended to the vibrations of atoms in crystals and to electromagnetic radiation in a cavity (see black-body radiation). Some of the results were consistent with experiment within certain conditions; for ex-ample, the principle predicts *Dulong and Petit's law for the specific heat capacities of solids, which was verified for most substances at the temperatures that were then attainable.
2 In the case of radiation the principle led to difficulties and Planck proposed the quantum theory ( 9) to overcome these. This led to extensive research, for example, the case of the ernst and Lindemann vacuum calorimeter to measure specific heat capacities at low temperatures. At the time of the first Solvay Conference (9l) leading scientists agreed that the equipartition principle was untenable in general, although it is an admissible approximation in certain cases, especially at high temperatures. α ( x, y or z) E pα mvα (Eq.) m α m v α p α α m r m m r r m m m r, + + mmr ω E m ( rω ) + m ( rω ) ( m + m ) mm µ reduced mass m + m µ r ω moment of inertia I mm I mr m m r r i i µ + i l rm r + mm r m r m + m r r I ω ω ω µ ω ω l E Iω (Eq.) I 9 (X, Y, Z)
3 (Eq.) la Ea I aω a, a X, Y, Z (Eq.3) I a I a l a a Z p l, m I, v ω m p E + Kq (Eq.4) m p K q q Asin( ω t), p mq maω cos( ω t), f Kq p mω q y By T By ( ) P By A exp (Eq.5) kt y y + By A dy kt exp (Eq.5) By + By By exp dy + kt By By P( By ) dy + By exp dy kt 3
4 β + ln exp( β By ) + dy, β kt (Eq.6) exp( β By )dy Laplace I 5 Laplace 33p x x n n+ a, e ax e dx ax dx ( n ) n+ n! n a +!! π a n+ ( n )!! ( n )( n 3)( n 5), ( )!! (Eq.6) d π d By ln ln d B β β β dβ, β kt (Eq.7) By (/)kt (/)kt 4
5 BJ ( )( ) ( J + ) BJ J + J + exp J kt ε BJ ( ) ( J + ) J + exp J kt d ln dβ J ( J + ) exp[ β BJ( J + ) ] K J ( J + ) exp[ β BJ( J + ) ] J xe, dx β β Bx β B d ε ln β kt dβ β kt exp( β By )dy β + + β B y x exp( β By ) dy exp( x )dx + β B exp( x )dx β + 5
6 ideal gas (Helmholtz) (Gibbs) extensive variables intensive variables E de S E S E V E V, S, S, V E ds + V E dv + V, S, S, V temperature, pressure d electrochemical potential chemical potential chemical potential µ + µ + + µ r r µ i i i equations of state 6
7 3/ 5/ e s v E S V, e E, v V s S, e v s s + + cr ln R ln e v 7
8 / c e v ( s s ) / cr ln e v e / c v s s e e exp v cr e e s cr T e v e crt c v cv P T v s s + + cr ln R ln T v P v s s cr ln ( c ) R ln P v T P s s + ( c + ) R ln + R ln T P h P s s + + ( c )ln R ln h P /( c+ ) P s s h h exp P ( c + ) R h h s c + ( ) R T h h RT v P ( c+ ) P P f crt st crt ln( T / T) RT ln( v / v) g ( c + ) RT ( c + ) RT ln( T / T) + RT ln( P / P) st 8
9 97 (5) g g + RT ln P g T P g ( c + ) R ln + R s s T T ln P RT / P v P ( c+ ) / c / c V S S E E exp V cr c R E E SE e se µ + e + e + Pv st g c c R c cr T V S + s + c R ln R ln in Eq (I6) T v T + V S s + c R ln R ln T v E c RT V V v v 9
10 T V S + s + c R ln R ln R ln T v / V / V V V s + crln ( T / T) + Rln ( v/ v) [ ] entropy of mixing S m Sm / sm R ln R xlnx G( TP,,, ) µ ( TP, ) + µ ( TP, ) + RT ln + RT ln + + µ ( T, P), µ ( T, P) G( T, P,, ) µ ( T, P) + µ ( T, P) RT + RT ln ln ln { / ( + / ) } ln, + + x, x, x + x + +
11 G µ ( T, P, x) µ ( T, P) + RTln x, x x G µ ( T, P, x) µ ( T, P) + RTlnx µ µ ( T, P, x) ( T, P) x RT µ ( T, P, x ) µ ( T, P) + RTlnx colligative Properties m M / M ( ) M m { m /[ / M ] } x [ / M ] /[ / M ] m / m m m m /[ / M ] k + m [ / M ] m m m mol Kg µ µ + RT ln k + RT ln m m ( ) / P.W. Atkins, Physical Chemistry, fourth edition, p8(99, Richard Clay Ltd) Herbert B. Callen, Thermodynamics and an Introduction to Thermostatics, Second edition (985, John Wiley Sons, Inc.)
12 ideal van der Waals fluid P R T BT CT DT v + ( ) v + ( ) v + ( ) v + 3 second virial coefficient third virial coefficient ideally f v T P f( T v) f T v RT BT ( ) CT ( ) DT ( ), ideal (, ) v v 3v f d( BT) d( CT ) d( DT) s sideal R T v dt v dt 3v dt db dc dd e eideal RT v dt v dt 3v dt s ideal, e ideal molar heat capacity at constant volume de Tds c v dt at v constant c v s f cv T T T T v v d ( BT) d ( CT) d ( DT) cv cv, ideal RT v 3 dt v dt 3v dt
13 3 ε 3 BT ( ) b ( R ) exp, b A kt π σ 3 Molecular Theory of Gases and Liquids (Hirschfelder, Curtiss & Bird, John Wiley & Sons, Inc., p.58) 3 BT ( ) b b R ε / kt ( ) squarewell potential Lennard-Jones (6-) potential:.8.56 (Hirschfelder et al. p59) BT ( )/ b CT ( )/ b exp( ε / kt ) σ, b, R, ε/ k (Hirschfelder et al. p6) σ( 6 3 m) b ( m ) ε / k( K) real gas van der Waals 873 3
14 P R T a or P a v v b RT v b v T +, ( / )( ) P R a R b b a T v / v v T v v v v T R v v b a b T v BT ( ) b a/ T, CT ( ) b ( ) 3 4π σ 3 ε b b 4 A, a b( R ) 3 k excluded volume effect 4 π σ 3 3 van der Waals ds T de P + T dv 4
15 s s v e e v P v T e e T v R a v T e e v b v T v a v e T v ( / v) T e ( e/ a) T v cr cr / a T e e/ a cr / a cr ( y) T e/ a+ / v e + a / v ideal van der Waals fluid P R acr T v b ev + av ds cr de a dv + R dv e+ a/ v v e+ a/ v v b crd ln( e + a / v) + Rd ln( v b) van der Waals e+ a/ v v b s s + crln + R ln e + a/ v v b 5
16 / c a v b s s a esv (, ) e + exp v v b cr v c a V b S s a E / / / e + exp v v b cr V van der Waals T v b s s + crln + R ln T v b P+ a/ v v b s s + crln + ( c+ ) Rln P + a/ v v b T P + a / v s s + + ( c ) R ln R ln T P + a / v van der Waals 6
17 general ideal gas simple ideal gas general ideal gas ernst theorem T e e c ( T ' ) dt ' + T v cv ( T) dv ds dt + R T v T c v ( T ) v s s + + dt R ln T T v T T cv ( T ) crln dt T T T T c v ( T ) v S + + s dt R ln R x ln x T T v 7
18 T E e + cv ( T ) dt T G µ µ RT[ φ T + P + x ] φ T ( ) ln ln e s T ( ) + ln P ln ( ) RT R + T RT R T T ' ' cv T dt T T c v ' ( T ) dt ' T ' partial molar Gibbs potential ( ) e c RT s φ ( T ) + ( + c )[ ln( T / T )] ln P RT R g g + RT ln P P Px ( ) g RT ( + c ) ln( T / T ) s [ ] ln P R 8
19 ν A stoichiometric coefficient d d ~ d ν ν d ~ d ~ ν d dg SdT + VdP + µ + µ + ~ ~ SdT + VdP + νµ d + νµ d + SdT + VdP + d ~ ν µ dg d νµ νµ heat of reaction chemical potential G H G TS G T T + P,,, 9
20 d dh ~ dg ~ dg ~ dh ~ d ~ d T ~ d d d T d P,,, dh ~ T ν µ d T dh d endothermic reaction exothermic reaction ν ln x ν ln P ν φ ( T) ln KT ( ) νφ( T) mass action law ν x P K( T) ν ν ν K( T ) ( Px ) P ( dh ~ T RT ν φ + RT ν ln P + RT ln ν ln x d T d ν µ RT ν φ ( T ) dt νµ dh RT d ln KT ( ) d dt van t Hoff relation
21 ln K ( T ) dh ~ + const RT d ν φ ( T ) G / RT dh / d H H ~ ~ ~,, a, b, + c ( ) ( ) A B C A B H c HC a HA b HB H / chc aha bhb H H ln K ( T ) + const RT G RT ln K, G H T S H S ln K + RT R C
22 Einstein Model Dulong-Petit law H m p m ω + q harmonic oscillator fixed points collective vibrational motion Debye
23 ε ( ν+ / ) ω h h ε ω ε n ω ω ω ω ω ω ω ω ω ( 3 + E / ω)! ( 3 + E / ω)! Ω ( 3 )!( E / ω)! ( 3)!( E / ω)! S kln Ω Stirling approximation ln( M!) Mln M M if ω ln Ω ( 3 + X) ln( 3 + X) 3 ln 3 X ln X 3 ( + Y) ln 3( + Y) ln3 Yln3Y 3 ( + Y) ln( + Y) YlnY 3 ln( + Y) + Yln( + / Y) e e e s 3R ln 3R ln e e e 3
24 e 3 A ω / T s/ e 3R e + ln T e e 3 A ω e exp( ω / kt ) 3Rexp( ω / kt ) ω c v [ exp( ω / kt ) ] kt ω / k ΘE Einstein cv 3Rf E ( Θ E / T ) x exp( x) f E () x [ exp( x) ] x x e x ( + x + x / + ) x e ( + x + x / + ) ( ) microcanonical ensemble ω ω ω 4
25 , S kln Ω microcanonical ensemble ω ω ω ε av n n ω exp n exp ( n ω / kt ) ( n ω / kt ) ex x x e + e + e + + x n e e x nx d nx e ne e x n dx n e ( ) x 5
26 ω ε av ω exp kt e 3 A ε av Debye 9 Θ D e Θ D + 3RTD 8 T 3 3 x ξ dξ D( x) 3 x ξ e Θ D 3Θ D / T cv 3R 4D T exp( Θ / T ) D Θ D Debye Debye 6
27 p h n n x y nz ε + + n 8 x, ny, nz 3,,, m m lx l y lz p ( ) hn hn x y hnz ε, p px, p y, pz,, m lx l y lz nx, ny, nz,,,,,, h / l, h / l, h / l ( ) x y h h h l x l y l z z 3 h, V V l x l y l z 3 V / h V Vdpxdp ydpz p dp 3 xdp ydpz 3 h h dxdydzdp xdpydpz 3 dxdydzdp dp dp / h x y z Landau Lifshitz Quantum Mechanics (third Edition,Pergamon Press) (48.7) 7
28 q qs p p s / π ( ) s E ε + ε + + ε ( P + p + + p ) m p i ( p xi, p yi, pzi ) pxi me ξi, pyi me ξ, p me i zi ξ + + i ξ + ξ + + ξ 3 me Γ ( E) ~ V Γ( E) h V 3 h ( me) 3 pi me i 3 / dp 3 ξi i x dp y dp z dp dξ dξ dξ x 3 dp y dp z 3 / (me) V C 3 3 h C 3 Wm. G. Hoover, Computational Statistical Mechanics (Studies in Modern Thermodynamics, Elsevier, 99) p.7 8
29 + + + / dx dx dx exp( r ) + dx exp( x ) π d( C R )/ dr C R d( C R ) C R dr π / C Γ ( r ) dr C r exp( r ) C r exp ( / ) / C Γ + / C π / Γ + Γ( n) n! n n n Γ π 3 / C π / Γ( 3 / + ) 3 3 3! / e ! Γ +! Γ( 3 / + ) 3 ±! 3 ± / e 3 ± 3 3 e 3 / C 3 dr 9
30 3 / πe C 3 3 Γ ~ ( E) 3 / ~ 4πemE Γ( E) V 3h ~ 3 / ~ 3 4πem 3h 3 / E ( E de) E V de Γ( ) Γ + 3 / 3 4πem 3 / E V 3h! ( / e) 3 / 3 / 3 4πm 5 / E V Ω( E, V, ) e 3h 5 3 3h 3 E V S( E, V, ) k ln + k ln + k ln 4πm 5 3 3h S( E, V, ) R ( ln ) A ln 4πm 3 E V + R ln + R ln A 6. 3 h (Js) 5 R ln 3 3h 3 ln 4.458R + R ln M 4 m π ( A ) w 4.458R 37.66(JK - ) 3
31 canonical ensemble S( E, V, ) kln Ω( E, V, ) thermal reservoir subsystem tot tot tot res tot res tot res tot Ω E E Ω E res ( tot ) tot ( tot ) total system 3
32 Ω res ( Etot E ) f Ω E tot ( ) tot f { S ( E E )/ k} exp res tot exp { S ( E )/ k} tot tot S E S E + S E E ( ) ( ) ( ) tot tot res ( Etot E ) S ( E E ) S ( E E + E E ) res tot res tot S res tot S ( ) ( ) res E E E E S res ( Etot E) + tot E E S res ( Etot E) + T f exp{ β[ E TS( E) ] βe }, β kt Helmholtz βf βe f e e exp βf βe f e e canonical partition sum Z e E β β e F Z, F kt ln Z ( ) 3
33 canonical ensemble f βe e / βe e - Maxwell-Boltzmann distribution ν ν Eν Etot Eν ν! Ω( ν, ν, ) ν! ν! () () () () ( n) ( n) Ω Ω( ν, ν, ) + Ω( ν, ν, ) + + Ω( ν, ν, ) + S k ln Ω( ν, ν, ) 33
34 W! W!! n +! W () n W n! + n! n ( )( ) ( ( n ) ) ( + )( + ) ( + n ) W ln Wn ( ) lnw + ln( ) + ln( ) + + ln( ( n ) ) ln( + ) ln( + ) ln( + n ) n / lnw n ln W e ( ) W( n) W exp( n / ) n / x Stirling 34
35 ( )! / e W W ( / e)!! Stirling! e π W π /π lnw ln + ln ln π ln ln ln ln / ln ln ln ln ln Stirling Stirling S / k νln ν/ e νln ν / e νln ν / e δ ( S / k) ν + δν ln ν + δν ν lnν { ( ) ( ) } ( lnν + ) δν δν E δν ( ln ν ) δν L 35
36 ( lnν + α + βe ), ( lnν + α + βe ) ( ln ν + α + βe ) δν L lnν + α + βe, ν exp α βe ( ) f exp βe / exp βe ( ) ( ) E f E ln exp( βe ) β F F F E F TS F T T T V T T kt kt β, F kt ln ( ) exp βe kt F( T, V, ) kt ln Z( T, V, ) ln Z β 36
37 S( E, V, ) kln Ω( E, V, ), β Ω βe Z( T V, ) Z( ) ( E, V, ) e de βe Z T, V, Ω E, V, e ( ) ( ) Laplace transform Inverse Laplace transform c+ i c i pe Ω ( E) Ω( E V, ) Z( p) e dp, d E E E f ln Z( β ) dβ 37
38 ε ( p + p + p ) x y z m dxdydzdp xdpydpz dxdydzdp dp dp 3 / h x y z transl dxdydz dpxdp ydpz exp β px + p y + p z / 3 z [ ( ) m] h V 3 h πmkt [ ] 3 / π α / e αx dx ( α ) ( z ) transl Z z V 3 / transl πmkt 3!! h 3 / V πmkt F kt ln Z kt ln kt h E Z kt β ln 3 E F 5 V 3 π S k + k ln + ln mkt T h 38
untitled
 1 Physical Chemistry I (Basic Chemical Thermodynamics) [I] [II] [III] [IV] Introduction Energy(The First Law of Thermodynamics) Work Heat Capacity C p and C v Adiabatic Change Exact(=Perfect) Differential
1 Physical Chemistry I (Basic Chemical Thermodynamics) [I] [II] [III] [IV] Introduction Energy(The First Law of Thermodynamics) Work Heat Capacity C p and C v Adiabatic Change Exact(=Perfect) Differential
5 5.1 E 1, E 2 N 1, N 2 E tot N tot E tot = E 1 + E 2, N tot = N 1 + N 2 S 1 (E 1, N 1 ), S 2 (E 2, N 2 ) E 1, E 2 S tot = S 1 + S 2 2 S 1 E 1 = S 2 E
 5 5.1 E 1, E 2 N 1, N 2 E tot N tot E tot = E 1 + E 2, N tot = N 1 + N 2 S 1 (E 1, N 1 ), S 2 (E 2, N 2 ) E 1, E 2 S tot = S 1 + S 2 2 S 1 E 1 = S 2 E 2, S 1 N 1 = S 2 N 2 2 (chemical potential) µ S N
5 5.1 E 1, E 2 N 1, N 2 E tot N tot E tot = E 1 + E 2, N tot = N 1 + N 2 S 1 (E 1, N 1 ), S 2 (E 2, N 2 ) E 1, E 2 S tot = S 1 + S 2 2 S 1 E 1 = S 2 E 2, S 1 N 1 = S 2 N 2 2 (chemical potential) µ S N
nm (T = K, p = kP a (1atm( )), 1bar = 10 5 P a = atm) 1 ( ) m / m
 .1 1nm (T = 73.15K, p = 101.35kP a (1atm( )), 1bar = 10 5 P a = 0.9863atm) 1 ( ).413968 10 3 m 3 1 37. 1/3 3.34.414 10 3 m 3 6.0 10 3 = 3.7 (109 ) 3 (nm) 3 10 6 = 3.7 10 1 (nm) 3 = (3.34nm) 3 ( P = nrt,
.1 1nm (T = 73.15K, p = 101.35kP a (1atm( )), 1bar = 10 5 P a = 0.9863atm) 1 ( ).413968 10 3 m 3 1 37. 1/3 3.34.414 10 3 m 3 6.0 10 3 = 3.7 (109 ) 3 (nm) 3 10 6 = 3.7 10 1 (nm) 3 = (3.34nm) 3 ( P = nrt,
30
 3 ............................................2 2...........................................2....................................2.2...................................2.3..............................
3 ............................................2 2...........................................2....................................2.2...................................2.3..............................
Microsoft Word - ●ipho-text3目次
 国際物理オリンピック 研修用テキスト Ⅲ 熱物理 相対論 量子力学 特定非営利活動法人物理オリンピック日本委員会 1 1.1 1 1. 1.3 3 1.4 4 1.5 6 1.6 7 1.7 9 11.1 11. 0.3 1 6 3.1 6 3. -9 3.3 - -- 31 3.4 --33 39 4.1 39 4. 40 4.3 4 4.4 44 4.5 47 5 5.1 5 5. 5 5.3
国際物理オリンピック 研修用テキスト Ⅲ 熱物理 相対論 量子力学 特定非営利活動法人物理オリンピック日本委員会 1 1.1 1 1. 1.3 3 1.4 4 1.5 6 1.6 7 1.7 9 11.1 11. 0.3 1 6 3.1 6 3. -9 3.3 - -- 31 3.4 --33 39 4.1 39 4. 40 4.3 4 4.4 44 4.5 47 5 5.1 5 5. 5 5.3
master.dvi
 4 Maxwell- Boltzmann N 1 4.1 T R R 5 R (Heat Reservor) S E R 20 E 4.2 E E R E t = E + E R E R Ω R (E R ) S R (E R ) Ω R (E R ) = exp[s R (E R )/k] E, E E, E E t E E t E exps R (E t E) exp S R (E t E )
4 Maxwell- Boltzmann N 1 4.1 T R R 5 R (Heat Reservor) S E R 20 E 4.2 E E R E t = E + E R E R Ω R (E R ) S R (E R ) Ω R (E R ) = exp[s R (E R )/k] E, E E, E E t E E t E exps R (E t E) exp S R (E t E )
all.dvi
 I 1 Density Matrix 1.1 ( (Observable) Ô :ensemble ensemble average) Ô en =Tr ˆρ en Ô ˆρ en Tr  n, n =, 1,, Tr  = n n  n Tr  I w j j ( j =, 1,, ) ˆρ en j w j j ˆρ en = j w j j j Ô en = j w j j Ô j emsemble
I 1 Density Matrix 1.1 ( (Observable) Ô :ensemble ensemble average) Ô en =Tr ˆρ en Ô ˆρ en Tr  n, n =, 1,, Tr  = n n  n Tr  I w j j ( j =, 1,, ) ˆρ en j w j j ˆρ en = j w j j j Ô en = j w j j Ô j emsemble
// //( ) (Helmholtz, Hermann Ludwig Ferdinand von: ) [ ]< 35, 36 > δq =0 du
![// //( ) (Helmholtz, Hermann Ludwig Ferdinand von: ) [ ]< 35, 36 > δq =0 du // //( ) (Helmholtz, Hermann Ludwig Ferdinand von: ) [ ]< 35, 36 > δq =0 du](/thumbs/91/105254510.jpg) 2 2.1 1 [ 1 ]< 33, 34 > 1 (the first law of thermodynamics) U du = δw + δq (1) (internal energy)u (work)w δw rev = PdV (2) P (heat)q 1 1. U ( U ) 2. 1 (perpetuum mobile) 3. du 21 // //( ) (Helmholtz, Hermann
2 2.1 1 [ 1 ]< 33, 34 > 1 (the first law of thermodynamics) U du = δw + δq (1) (internal energy)u (work)w δw rev = PdV (2) P (heat)q 1 1. U ( U ) 2. 1 (perpetuum mobile) 3. du 21 // //( ) (Helmholtz, Hermann
( ) ,
 II 2007 4 0. 0 1 0 2 ( ) 0 3 1 2 3 4, - 5 6 7 1 1 1 1 1) 2) 3) 4) ( ) () H 2.79 10 10 He 2.72 10 9 C 1.01 10 7 N 3.13 10 6 O 2.38 10 7 Ne 3.44 10 6 Mg 1.076 10 6 Si 1 10 6 S 5.15 10 5 Ar 1.01 10 5 Fe 9.00
II 2007 4 0. 0 1 0 2 ( ) 0 3 1 2 3 4, - 5 6 7 1 1 1 1 1) 2) 3) 4) ( ) () H 2.79 10 10 He 2.72 10 9 C 1.01 10 7 N 3.13 10 6 O 2.38 10 7 Ne 3.44 10 6 Mg 1.076 10 6 Si 1 10 6 S 5.15 10 5 Ar 1.01 10 5 Fe 9.00
1 9 v.0.1 c (2016/10/07) Minoru Suzuki T µ 1 (7.108) f(e ) = 1 e β(e µ) 1 E 1 f(e ) (Bose-Einstein distribution function) *1 (8.1) (9.1)
 1 9 v..1 c (216/1/7) Minoru Suzuki 1 1 9.1 9.1.1 T µ 1 (7.18) f(e ) = 1 e β(e µ) 1 E 1 f(e ) (Bose-Einstein distribution function) *1 (8.1) (9.1) E E µ = E f(e ) E µ (9.1) µ (9.2) µ 1 e β(e µ) 1 f(e )
1 9 v..1 c (216/1/7) Minoru Suzuki 1 1 9.1 9.1.1 T µ 1 (7.18) f(e ) = 1 e β(e µ) 1 E 1 f(e ) (Bose-Einstein distribution function) *1 (8.1) (9.1) E E µ = E f(e ) E µ (9.1) µ (9.2) µ 1 e β(e µ) 1 f(e )
September 25, ( ) pv = nrt (T = t( )) T: ( : (K)) : : ( ) e.g. ( ) ( ): 1
 September 25, 2017 1 1.1 1.2 p = nr = 273.15 + t : : K : 1.3 1.3.1 : e.g. 1.3.2 : 1 intensive variable e.g. extensive variable e.g. 1.3.3 Equation of State e.g. p = nr X = A 2 2.1 2.1.1 Quantity of Heat
September 25, 2017 1 1.1 1.2 p = nr = 273.15 + t : : K : 1.3 1.3.1 : e.g. 1.3.2 : 1 intensive variable e.g. extensive variable e.g. 1.3.3 Equation of State e.g. p = nr X = A 2 2.1 2.1.1 Quantity of Heat
iBookBob:Users:bob:Documents:CurrentData:flMŠÍ…e…L…X…g:Statistics.dvi
 4 4 9............................................... 3.3......................... 4.4................. 5.5............................ 7 9..................... 9.............................3................................4..........................5.............................6...........................
4 4 9............................................... 3.3......................... 4.4................. 5.5............................ 7 9..................... 9.............................3................................4..........................5.............................6...........................
現代物理化学 2-1(9)16.ppt
 --- S A, G U S S ds = d 'Q r / ΔS = S S = ds =,r,r d 'Q r r S -- ds = d 'Q r / ΔS = S S = ds =,r,r d 'Q r r d Q r e = P e = P ΔS d 'Q / e (d'q / e ) --3,e Q W Q (> 0),e e ΔU = Q + W = (Q + Q ) + W = 0
--- S A, G U S S ds = d 'Q r / ΔS = S S = ds =,r,r d 'Q r r S -- ds = d 'Q r / ΔS = S S = ds =,r,r d 'Q r r d Q r e = P e = P ΔS d 'Q / e (d'q / e ) --3,e Q W Q (> 0),e e ΔU = Q + W = (Q + Q ) + W = 0
/ Christopher Essex Radiation and the Violation of Bilinearity in the Thermodynamics of Irreversible Processes, Planet.Space Sci.32 (1984) 1035 Radiat
 / Christopher Essex Radiation and the Violation of Bilinearity in the Thermodynamics of Irreversible Processes, Planet.Space Sci.32 (1984) 1035 Radiation and the Continuing Failure of the Bilinear Formalism,
/ Christopher Essex Radiation and the Violation of Bilinearity in the Thermodynamics of Irreversible Processes, Planet.Space Sci.32 (1984) 1035 Radiation and the Continuing Failure of the Bilinear Formalism,
V(x) m e V 0 cos x π x π V(x) = x < π, x > π V 0 (i) x = 0 (V(x) V 0 (1 x 2 /2)) n n d 2 f dξ 2ξ d f 2 dξ + 2n f = 0 H n (ξ) (ii) H
 199 1 1 199 1 1. Vx) m e V cos x π x π Vx) = x < π, x > π V i) x = Vx) V 1 x /)) n n d f dξ ξ d f dξ + n f = H n ξ) ii) H n ξ) = 1) n expξ ) dn dξ n exp ξ )) H n ξ)h m ξ) exp ξ )dξ = π n n!δ n,m x = Vx)
199 1 1 199 1 1. Vx) m e V cos x π x π Vx) = x < π, x > π V i) x = Vx) V 1 x /)) n n d f dξ ξ d f dξ + n f = H n ξ) ii) H n ξ) = 1) n expξ ) dn dξ n exp ξ )) H n ξ)h m ξ) exp ξ )dξ = π n n!δ n,m x = Vx)
QMI_10.dvi
 ... black body radiation black body black body radiation Gustav Kirchhoff 859 895 W. Wien O.R. Lummer cavity radiation ν ν +dν f T (ν) f T (ν)dν = 8πν2 c 3 kt dν (Rayleigh Jeans) (.) f T (ν) spectral energy
... black body radiation black body black body radiation Gustav Kirchhoff 859 895 W. Wien O.R. Lummer cavity radiation ν ν +dν f T (ν) f T (ν)dν = 8πν2 c 3 kt dν (Rayleigh Jeans) (.) f T (ν) spectral energy
0201
 2018 10 17 2019 9 19 SI J cal 1mL 1ºC 1999 cal nutrition facts label calories cal kcal 1 cal = 4.184 J heat capacity 1 K 1 J K 1 mol molar heat capacity J K mol (specific heat specific heat capacity) 1
2018 10 17 2019 9 19 SI J cal 1mL 1ºC 1999 cal nutrition facts label calories cal kcal 1 cal = 4.184 J heat capacity 1 K 1 J K 1 mol molar heat capacity J K mol (specific heat specific heat capacity) 1
3.2 [ ]< 86, 87 > ( ) T = U V,N,, du = TdS PdV + µdn +, (3) P = U V S,N,, µ = U N. (4) S,V,, ( ) ds = 1 T du + P T dv µ dn +, (5) T 1 T = P U V,N,, T
![3.2 [ ]< 86, 87 > ( ) T = U V,N,, du = TdS PdV + µdn +, (3) P = U V S,N,, µ = U N. (4) S,V,, ( ) ds = 1 T du + P T dv µ dn +, (5) T 1 T = P U V,N,, T 3.2 [ ]< 86, 87 > ( ) T = U V,N,, du = TdS PdV + µdn +, (3) P = U V S,N,, µ = U N. (4) S,V,, ( ) ds = 1 T du + P T dv µ dn +, (5) T 1 T = P U V,N,, T](/thumbs/82/86676506.jpg) 3 3.1 [ ]< 85, 86 > ( ) ds > 0. (1) dt ds dt =0, S = S max. (2) ( δq 1 = TdS 1 =0) (δw 1 < 0) (du 1 < 0) (δq 2 > 0) (ds = ds 2 = TδQ 2 > 0) 39 3.2 [ ]< 86, 87 > ( ) T = U V,N,, du = TdS PdV + µdn +, (3)
3 3.1 [ ]< 85, 86 > ( ) ds > 0. (1) dt ds dt =0, S = S max. (2) ( δq 1 = TdS 1 =0) (δw 1 < 0) (du 1 < 0) (δq 2 > 0) (ds = ds 2 = TδQ 2 > 0) 39 3.2 [ ]< 86, 87 > ( ) T = U V,N,, du = TdS PdV + µdn +, (3)
70 5. (isolated system) ( ) E N (closed system) N T (open system) (homogeneous) (heterogeneous) (phase) (phase boundary) (grain) (grain boundary) 5. 1
 5 0 1 2 3 (Carnot) (Clausius) 2 5. 1 ( ) ( ) ( ) ( ) 5. 1. 1 (system) 1) 70 5. (isolated system) ( ) E N (closed system) N T (open system) (homogeneous) (heterogeneous) (phase) (phase boundary) (grain)
5 0 1 2 3 (Carnot) (Clausius) 2 5. 1 ( ) ( ) ( ) ( ) 5. 1. 1 (system) 1) 70 5. (isolated system) ( ) E N (closed system) N T (open system) (homogeneous) (heterogeneous) (phase) (phase boundary) (grain)
磁性物理学 - 遷移金属化合物磁性のスピンゆらぎ理論
 email: takahash@sci.u-hyogo.ac.jp May 14, 2009 Outline 1. 2. 3. 4. 5. 6. 2 / 262 Today s Lecture: Mode-mode Coupling Theory 100 / 262 Part I Effects of Non-linear Mode-Mode Coupling Effects of Non-linear
email: takahash@sci.u-hyogo.ac.jp May 14, 2009 Outline 1. 2. 3. 4. 5. 6. 2 / 262 Today s Lecture: Mode-mode Coupling Theory 100 / 262 Part I Effects of Non-linear Mode-Mode Coupling Effects of Non-linear
Maxwell
 I 2018 12 13 0 4 1 6 1.1............................ 6 1.2 Maxwell......................... 8 1.3.......................... 9 1.4..................... 11 1.5..................... 12 2 13 2.1...................
I 2018 12 13 0 4 1 6 1.1............................ 6 1.2 Maxwell......................... 8 1.3.......................... 9 1.4..................... 11 1.5..................... 12 2 13 2.1...................
08 p Boltzmann I P ( ) principle of equal probability P ( ) g ( )g ( 0 ) (4 89) (4 88) eq II 0 g ( 0 ) 0 eq Taylor eq (4 90) g P ( ) g ( ) g ( 0
 08 p. 8 4 k B log g() S() k B : Boltzmann T T S k B g g heat bath, thermal reservoir... 4. I II II System I System II II I I 0 + 0 const. (4 85) g( 0 ) g ( )g ( ) g ( )g ( 0 ) (4 86) g ( )g ( 0 ) 0 (4
08 p. 8 4 k B log g() S() k B : Boltzmann T T S k B g g heat bath, thermal reservoir... 4. I II II System I System II II I I 0 + 0 const. (4 85) g( 0 ) g ( )g ( ) g ( )g ( 0 ) (4 86) g ( )g ( 0 ) 0 (4
. ev=,604k m 3 Debye ɛ 0 kt e λ D = n e n e Ze 4 ln Λ ν ei = 5.6π / ɛ 0 m/ e kt e /3 ν ei v e H + +e H ev Saha x x = 3/ πme kt g i g e n
 003...............................3 Debye................. 3.4................ 3 3 3 3. Larmor Cyclotron... 3 3................ 4 3.3.......... 4 3.3............ 4 3.3...... 4 3.3.3............ 5 3.4.........
003...............................3 Debye................. 3.4................ 3 3 3 3. Larmor Cyclotron... 3 3................ 4 3.3.......... 4 3.3............ 4 3.3...... 4 3.3.3............ 5 3.4.........
I-2 (100 ) (1) y(x) y dy dx y d2 y dx 2 (a) y + 2y 3y = 9e 2x (b) x 2 y 6y = 5x 4 (2) Bernoulli B n (n = 0, 1, 2,...) x e x 1 = n=0 B 0 B 1 B 2 (3) co
 16 I ( ) (1) I-1 I-2 I-3 (2) I-1 ( ) (100 ) 2l x x = 0 y t y(x, t) y(±l, t) = 0 m T g y(x, t) l y(x, t) c = 2 y(x, t) c 2 2 y(x, t) = g (A) t 2 x 2 T/m (1) y 0 (x) y 0 (x) = g c 2 (l2 x 2 ) (B) (2) (1)
16 I ( ) (1) I-1 I-2 I-3 (2) I-1 ( ) (100 ) 2l x x = 0 y t y(x, t) y(±l, t) = 0 m T g y(x, t) l y(x, t) c = 2 y(x, t) c 2 2 y(x, t) = g (A) t 2 x 2 T/m (1) y 0 (x) y 0 (x) = g c 2 (l2 x 2 ) (B) (2) (1)
(Compton Scattering) Beaming 1 exp [i (k x ωt)] k λ k = 2π/λ ω = 2πν k = ω/c k x ωt ( ω ) k α c, k k x ωt η αβ k α x β diag( + ++) x β = (ct, x) O O x
![(Compton Scattering) Beaming 1 exp [i (k x ωt)] k λ k = 2π/λ ω = 2πν k = ω/c k x ωt ( ω ) k α c, k k x ωt η αβ k α x β diag( + ++) x β = (ct, x) O O x (Compton Scattering) Beaming 1 exp [i (k x ωt)] k λ k = 2π/λ ω = 2πν k = ω/c k x ωt ( ω ) k α c, k k x ωt η αβ k α x β diag( + ++) x β = (ct, x) O O x](/thumbs/104/162595168.jpg) Compton Scattering Beaming exp [i k x ωt] k λ k π/λ ω πν k ω/c k x ωt ω k α c, k k x ωt η αβ k α x β diag + ++ x β ct, x O O x O O v k α k α β, γ k γ k βk, k γ k + βk k γ k k, k γ k + βk 3 k k 4 k 3 k
Compton Scattering Beaming exp [i k x ωt] k λ k π/λ ω πν k ω/c k x ωt ω k α c, k k x ωt η αβ k α x β diag + ++ x β ct, x O O x O O v k α k α β, γ k γ k βk, k γ k + βk k γ k k, k γ k + βk 3 k k 4 k 3 k
 B 1 B.1.......................... 1 B.1.1................. 1 B.1.2................. 2 B.2........................... 5 B.2.1.......................... 5 B.2.2.................. 6 B.2.3..................
B 1 B.1.......................... 1 B.1.1................. 1 B.1.2................. 2 B.2........................... 5 B.2.1.......................... 5 B.2.2.................. 6 B.2.3..................
1 (Berry,1975) 2-6 p (S πr 2 )p πr 2 p 2πRγ p p = 2γ R (2.5).1-1 : : : : ( ).2 α, β α, β () X S = X X α X β (.1) 1 2
 2005 9/8-11 2 2.2 ( 2-5) γ ( ) γ cos θ 2πr πρhr 2 g h = 2γ cos θ ρgr (2.1) γ = ρgrh (2.2) 2 cos θ θ cos θ = 1 (2.2) γ = 1 ρgrh (2.) 2 2. p p ρgh p ( ) p p = p ρgh (2.) h p p = 2γ r 1 1 (Berry,1975) 2-6
2005 9/8-11 2 2.2 ( 2-5) γ ( ) γ cos θ 2πr πρhr 2 g h = 2γ cos θ ρgr (2.1) γ = ρgrh (2.2) 2 cos θ θ cos θ = 1 (2.2) γ = 1 ρgrh (2.) 2 2. p p ρgh p ( ) p p = p ρgh (2.) h p p = 2γ r 1 1 (Berry,1975) 2-6
6 2 T γ T B (6.4) (6.1) [( d nm + 3 ] 2 nt B )a 3 + nt B da 3 = 0 (6.9) na 3 = T B V 3/2 = T B V γ 1 = const. or T B a 2 = const. (6.10) H 2 = 8π kc2
![6 2 T γ T B (6.4) (6.1) [( d nm + 3 ] 2 nt B )a 3 + nt B da 3 = 0 (6.9) na 3 = T B V 3/2 = T B V γ 1 = const. or T B a 2 = const. (6.10) H 2 = 8π kc2 6 2 T γ T B (6.4) (6.1) [( d nm + 3 ] 2 nt B )a 3 + nt B da 3 = 0 (6.9) na 3 = T B V 3/2 = T B V γ 1 = const. or T B a 2 = const. (6.10) H 2 = 8π kc2](/thumbs/92/109118076.jpg) 1 6 6.1 (??) (P = ρ rad /3) ρ rad T 4 d(ρv ) + PdV = 0 (6.1) dρ rad ρ rad + 4 da a = 0 (6.2) dt T + da a = 0 T 1 a (6.3) ( ) n ρ m = n (m + 12 ) m v2 = n (m + 32 ) T, P = nt (6.4) (6.1) d [(nm + 32 ] )a
1 6 6.1 (??) (P = ρ rad /3) ρ rad T 4 d(ρv ) + PdV = 0 (6.1) dρ rad ρ rad + 4 da a = 0 (6.2) dt T + da a = 0 T 1 a (6.3) ( ) n ρ m = n (m + 12 ) m v2 = n (m + 32 ) T, P = nt (6.4) (6.1) d [(nm + 32 ] )a
The Physics of Atmospheres CAPTER :
 The Physics of Atmospheres CAPTER 4 1 4 2 41 : 2 42 14 43 17 44 25 45 27 46 3 47 31 48 32 49 34 41 35 411 36 maintex 23/11/28 The Physics of Atmospheres CAPTER 4 2 4 41 : 2 1 σ 2 (21) (22) k I = I exp(
The Physics of Atmospheres CAPTER 4 1 4 2 41 : 2 42 14 43 17 44 25 45 27 46 3 47 31 48 32 49 34 41 35 411 36 maintex 23/11/28 The Physics of Atmospheres CAPTER 4 2 4 41 : 2 1 σ 2 (21) (22) k I = I exp(
δf = δn I [ ( FI (N I ) N I ) T,V δn I [ ( FI N I ( ) F N T,V ( ) FII (N N I ) + N I ) ( ) FII T,V N II T,V T,V ] ] = 0 = 0 (8.2) = µ (8.3) G
![δf = δn I [ ( FI (N I ) N I ) T,V δn I [ ( FI N I ( ) F N T,V ( ) FII (N N I ) + N I ) ( ) FII T,V N II T,V T,V ] ] = 0 = 0 (8.2) = µ (8.3) G δf = δn I [ ( FI (N I ) N I ) T,V δn I [ ( FI N I ( ) F N T,V ( ) FII (N N I ) + N I ) ( ) FII T,V N II T,V T,V ] ] = 0 = 0 (8.2) = µ (8.3) G](/thumbs/91/106215968.jpg) 8 ( ) 8. 1 ( ) F F = F I (N I, T, V I ) + F II (N II, T, V II ) (8.1) F δf = δn I [ ( FI (N I ) N I 8. 1 111 ) T,V δn I [ ( FI N I ( ) F N T,V ( ) FII (N N I ) + N I ) ( ) FII T,V N II T,V T,V ] ] = 0
8 ( ) 8. 1 ( ) F F = F I (N I, T, V I ) + F II (N II, T, V II ) (8.1) F δf = δn I [ ( FI (N I ) N I 8. 1 111 ) T,V δn I [ ( FI N I ( ) F N T,V ( ) FII (N N I ) + N I ) ( ) FII T,V N II T,V T,V ] ] = 0
Microsoft Word - 11問題表紙(選択).docx
 A B A.70g/cm 3 B.74g/cm 3 B C 70at% %A C B at% 80at% %B 350 C γ δ y=00 x-y ρ l S ρ C p k C p ρ C p T ρ l t l S S ξ S t = ( k T ) ξ ( ) S = ( k T) ( ) t y ξ S ξ / t S v T T / t = v T / y 00 x v S dy dx
A B A.70g/cm 3 B.74g/cm 3 B C 70at% %A C B at% 80at% %B 350 C γ δ y=00 x-y ρ l S ρ C p k C p ρ C p T ρ l t l S S ξ S t = ( k T ) ξ ( ) S = ( k T) ( ) t y ξ S ξ / t S v T T / t = v T / y 00 x v S dy dx
m d2 x = kx αẋ α > 0 (3.5 dt2 ( de dt = d dt ( 1 2 mẋ kx2 = mẍẋ + kxẋ = (mẍ + kxẋ = αẋẋ = αẋ 2 < 0 (3.6 Joule Joule 1843 Joule ( A B (> A ( 3-2
 3 3.1 ( 1 m d2 x(t dt 2 = kx(t k = (3.1 d 2 x dt 2 = ω2 x, ω = x(t = 0, ẋ(0 = v 0 k m (3.2 x = v 0 ω sin ωt (ẋ = v 0 cos ωt (3.3 E = 1 2 mẋ2 + 1 2 kx2 = 1 2 mv2 0 cos 2 ωt + 1 2 k v2 0 ω 2 sin2 ωt = 1
3 3.1 ( 1 m d2 x(t dt 2 = kx(t k = (3.1 d 2 x dt 2 = ω2 x, ω = x(t = 0, ẋ(0 = v 0 k m (3.2 x = v 0 ω sin ωt (ẋ = v 0 cos ωt (3.3 E = 1 2 mẋ2 + 1 2 kx2 = 1 2 mv2 0 cos 2 ωt + 1 2 k v2 0 ω 2 sin2 ωt = 1
4. ϵ(ν, T ) = c 4 u(ν, T ) ϵ(ν, T ) T ν π4 Planck dx = 0 e x 1 15 U(T ) x 3 U(T ) = σt 4 Stefan-Boltzmann σ 2π5 k 4 15c 2 h 3 = W m 2 K 4 5.
 A 1. Boltzmann Planck u(ν, T )dν = 8πh ν 3 c 3 kt 1 dν h 6.63 10 34 J s Planck k 1.38 10 23 J K 1 Boltzmann u(ν, T ) T ν e hν c = 3 10 8 m s 1 2. Planck λ = c/ν Rayleigh-Jeans u(ν, T )dν = 8πν2 kt dν c
A 1. Boltzmann Planck u(ν, T )dν = 8πh ν 3 c 3 kt 1 dν h 6.63 10 34 J s Planck k 1.38 10 23 J K 1 Boltzmann u(ν, T ) T ν e hν c = 3 10 8 m s 1 2. Planck λ = c/ν Rayleigh-Jeans u(ν, T )dν = 8πν2 kt dν c
I ( ) 2019
 I ( ) 2019 i 1 I,, III,, 1,,,, III,,,, (1 ) (,,, ), :...,, : NHK... NHK, (YouTube ),!!, manaba http://pen.envr.tsukuba.ac.jp/lec/physics/,, Richard Feynman Lectures on Physics Addison-Wesley,,,, x χ,
I ( ) 2019 i 1 I,, III,, 1,,,, III,,,, (1 ) (,,, ), :...,, : NHK... NHK, (YouTube ),!!, manaba http://pen.envr.tsukuba.ac.jp/lec/physics/,, Richard Feynman Lectures on Physics Addison-Wesley,,,, x χ,
4.6 (E i = ε, ε + ) T Z F Z = e βε + e β(ε+ ) = e βε (1 + e β ) F = kt log Z = kt log[e βε (1 + e β )] = ε kt ln(1 + e β ) (4.18) F (T ) S = T = k = k
![4.6 (E i = ε, ε + ) T Z F Z = e βε + e β(ε+ ) = e βε (1 + e β ) F = kt log Z = kt log[e βε (1 + e β )] = ε kt ln(1 + e β ) (4.18) F (T ) S = T = k = k 4.6 (E i = ε, ε + ) T Z F Z = e βε + e β(ε+ ) = e βε (1 + e β ) F = kt log Z = kt log[e βε (1 + e β )] = ε kt ln(1 + e β ) (4.18) F (T ) S = T = k = k](/thumbs/94/119235351.jpg) 4.6 (E i = ε, ε + ) T Z F Z = e ε + e (ε+ ) = e ε ( + e ) F = kt log Z = kt loge ε ( + e ) = ε kt ln( + e ) (4.8) F (T ) S = T = k = k ln( + e ) + kt e + e kt 2 + e ln( + e ) + kt (4.20) /kt T 0 = /k (4.20)
4.6 (E i = ε, ε + ) T Z F Z = e ε + e (ε+ ) = e ε ( + e ) F = kt log Z = kt loge ε ( + e ) = ε kt ln( + e ) (4.8) F (T ) S = T = k = k ln( + e ) + kt e + e kt 2 + e ln( + e ) + kt (4.20) /kt T 0 = /k (4.20)
微粒子合成化学・講義
 http://www.tagen.tohoku.ac.jp/labo/muramatsu/mura/main.html E-mail: mura@tagen.tohoku.ac.jp 1 Derjaguin Landau Verway Overbeek B.V.Derjaguin and L.Landau;Acta Physicochim.,URSS, 14, 633 1941. E.J.W.Verwey
http://www.tagen.tohoku.ac.jp/labo/muramatsu/mura/main.html E-mail: mura@tagen.tohoku.ac.jp 1 Derjaguin Landau Verway Overbeek B.V.Derjaguin and L.Landau;Acta Physicochim.,URSS, 14, 633 1941. E.J.W.Verwey
微粒子合成化学・講義
 http://www.tagen.tohoku.ac.jp/labo/muramatsu/mura/main.html E-mail: mura@tagen.tohoku.ac.jp 1 2 1 mol/l KCl 3 4 Derjaguin Landau Verway Overbeek B.V.Derjaguin and L.Landau;Acta Physicochim.,URSS, 14, 633
http://www.tagen.tohoku.ac.jp/labo/muramatsu/mura/main.html E-mail: mura@tagen.tohoku.ac.jp 1 2 1 mol/l KCl 3 4 Derjaguin Landau Verway Overbeek B.V.Derjaguin and L.Landau;Acta Physicochim.,URSS, 14, 633
II
 II 28 5 31 3 I 5 1 7 1.1.......................... 7 1.1.1 ( )................ 7 1.1.2........................ 12 1.1.3................... 13 1.1.4 ( )................. 14 1.1.5................... 15
II 28 5 31 3 I 5 1 7 1.1.......................... 7 1.1.1 ( )................ 7 1.1.2........................ 12 1.1.3................... 13 1.1.4 ( )................. 14 1.1.5................... 15
H22環境地球化学4_化学平衡III_ ppt
 1 2 3 2009年度 環境地球化学 大河内 温度上昇による炭酸水の発泡 気泡 温度が高くなると 溶けきれなくなった 二酸化炭素が気泡として出てくる 4 2009年度 環境地球化学 圧力上昇による炭酸水の発泡 栓を開けると 瓶の中の圧力が急激に 小さくなるので 発泡する 大河内 5 CO 2 K H CO 2 H 2 O K H + 1 HCO 3- K 2 H + CO 3 2- (M) [CO
1 2 3 2009年度 環境地球化学 大河内 温度上昇による炭酸水の発泡 気泡 温度が高くなると 溶けきれなくなった 二酸化炭素が気泡として出てくる 4 2009年度 環境地球化学 圧力上昇による炭酸水の発泡 栓を開けると 瓶の中の圧力が急激に 小さくなるので 発泡する 大河内 5 CO 2 K H CO 2 H 2 O K H + 1 HCO 3- K 2 H + CO 3 2- (M) [CO
I ( ) 1 de Broglie 1 (de Broglie) p λ k h Planck ( Js) p = h λ = k (1) h 2π : Dirac k B Boltzmann ( J/K) T U = 3 2 k BT
 I (008 4 0 de Broglie (de Broglie p λ k h Planck ( 6.63 0 34 Js p = h λ = k ( h π : Dirac k B Boltzmann (.38 0 3 J/K T U = 3 k BT ( = λ m k B T h m = 0.067m 0 m 0 = 9. 0 3 kg GaAs( a T = 300 K 3 fg 07345
I (008 4 0 de Broglie (de Broglie p λ k h Planck ( 6.63 0 34 Js p = h λ = k ( h π : Dirac k B Boltzmann (.38 0 3 J/K T U = 3 k BT ( = λ m k B T h m = 0.067m 0 m 0 = 9. 0 3 kg GaAs( a T = 300 K 3 fg 07345
Laves A-B AB 2 MgCu 2 (C14) MgZn 2 (C15) MgNi 2 (C36) Laves VASP ZrCr 2 Laves VASP(Vienna Ab-initio Simulation Package) Laves Energy-Volume Quasi-Harm
 ZrCr 2 Laves 5633 2009 2 Laves A-B AB 2 MgCu 2 (C14) MgZn 2 (C15) MgNi 2 (C36) Laves VASP ZrCr 2 Laves VASP(Vienna Ab-initio Simulation Package) Laves Energy-Volume Quasi-Harmonic Energy-Volume Phonon-DOS
ZrCr 2 Laves 5633 2009 2 Laves A-B AB 2 MgCu 2 (C14) MgZn 2 (C15) MgNi 2 (C36) Laves VASP ZrCr 2 Laves VASP(Vienna Ab-initio Simulation Package) Laves Energy-Volume Quasi-Harmonic Energy-Volume Phonon-DOS
IA
 IA 31 4 11 1 1 4 1.1 Planck.............................. 4 1. Bohr.................................... 5 1.3..................................... 6 8.1................................... 8....................................
IA 31 4 11 1 1 4 1.1 Planck.............................. 4 1. Bohr.................................... 5 1.3..................................... 6 8.1................................... 8....................................
.2 ρ dv dt = ρk grad p + 3 η grad (divv) + η 2 v.3 divh = 0, rote + c H t = 0 dive = ρ, H = 0, E = ρ, roth c E t = c ρv E + H c t = 0 H c E t = c ρv T
 NHK 204 2 0 203 2 24 ( ) 7 00 7 50 203 2 25 ( ) 7 00 7 50 203 2 26 ( ) 7 00 7 50 203 2 27 ( ) 7 00 7 50 I. ( ν R n 2 ) m 2 n m, R = e 2 8πε 0 hca B =.09737 0 7 m ( ν = ) λ a B = 4πε 0ħ 2 m e e 2 = 5.2977
NHK 204 2 0 203 2 24 ( ) 7 00 7 50 203 2 25 ( ) 7 00 7 50 203 2 26 ( ) 7 00 7 50 203 2 27 ( ) 7 00 7 50 I. ( ν R n 2 ) m 2 n m, R = e 2 8πε 0 hca B =.09737 0 7 m ( ν = ) λ a B = 4πε 0ħ 2 m e e 2 = 5.2977
untitled
 2 : n =1, 2,, 10000 0.5125 0.51 0.5075 0.505 0.5025 0.5 0.4975 0.495 0 2000 4000 6000 8000 10000 2 weak law of large numbers 1. X 1,X 2,,X n 2. µ = E(X i ),i=1, 2,,n 3. σi 2 = V (X i ) σ 2,i=1, 2,,n ɛ>0
2 : n =1, 2,, 10000 0.5125 0.51 0.5075 0.505 0.5025 0.5 0.4975 0.495 0 2000 4000 6000 8000 10000 2 weak law of large numbers 1. X 1,X 2,,X n 2. µ = E(X i ),i=1, 2,,n 3. σi 2 = V (X i ) σ 2,i=1, 2,,n ɛ>0
5 1.2, 2, d a V a = M (1.2.1), M, a,,,,, Ω, V a V, V a = V + Ω r. (1.2.2), r i 1, i 2, i 3, i 1, i 2, i 3, A 2, A = 3 A n i n = n=1 da = 3 = n=1 3 n=1
 4 1 1.1 ( ) 5 1.2, 2, d a V a = M (1.2.1), M, a,,,,, Ω, V a V, V a = V + Ω r. (1.2.2), r i 1, i 2, i 3, i 1, i 2, i 3, A 2, A = 3 A n i n = n=1 da = 3 = n=1 3 n=1 da n i n da n i n + 3 A ni n n=1 3 n=1
4 1 1.1 ( ) 5 1.2, 2, d a V a = M (1.2.1), M, a,,,,, Ω, V a V, V a = V + Ω r. (1.2.2), r i 1, i 2, i 3, i 1, i 2, i 3, A 2, A = 3 A n i n = n=1 da = 3 = n=1 3 n=1 da n i n da n i n + 3 A ni n n=1 3 n=1
E 1/2 3/ () +3/2 +3/ () +1/2 +1/ / E [1] B (3.2) F E 4.1 y x E = (E x,, ) j y 4.1 E int = (, E y, ) j y = (Hall ef
![E 1/2 3/ () +3/2 +3/ () +1/2 +1/ / E [1] B (3.2) F E 4.1 y x E = (E x,, ) j y 4.1 E int = (, E y, ) j y = (Hall ef E 1/2 3/ () +3/2 +3/ () +1/2 +1/ / E [1] B (3.2) F E 4.1 y x E = (E x,, ) j y 4.1 E int = (, E y, ) j y = (Hall ef](/thumbs/81/83176619.jpg) 4 213 5 8 4.1.1 () f A exp( E/k B ) f E = A [ k B exp E ] = f k B k B = f (2 E /3n). 1 k B /2 σ = e 2 τ(e)d(e) 2E 3nf 3m 2 E de = ne2 τ E m (4.1) E E τ E = τe E = / τ(e)e 3/2 f de E 3/2 f de (4.2) f (3.2)
4 213 5 8 4.1.1 () f A exp( E/k B ) f E = A [ k B exp E ] = f k B k B = f (2 E /3n). 1 k B /2 σ = e 2 τ(e)d(e) 2E 3nf 3m 2 E de = ne2 τ E m (4.1) E E τ E = τe E = / τ(e)e 3/2 f de E 3/2 f de (4.2) f (3.2)
Hanbury-Brown Twiss (ver. 2.0) van Cittert - Zernike mutual coherence
 Hanbury-Brown Twiss (ver. 2.) 25 4 4 1 2 2 2 2.1 van Cittert - Zernike..................................... 2 2.2 mutual coherence................................. 4 3 Hanbury-Brown Twiss ( ) 5 3.1............................................
Hanbury-Brown Twiss (ver. 2.) 25 4 4 1 2 2 2 2.1 van Cittert - Zernike..................................... 2 2.2 mutual coherence................................. 4 3 Hanbury-Brown Twiss ( ) 5 3.1............................................
 215 11 13 1 2 1.1....................... 2 1.2.................... 2 1.3..................... 2 1.4...................... 3 1.5............... 3 1.6........................... 4 1.7.................. 4
215 11 13 1 2 1.1....................... 2 1.2.................... 2 1.3..................... 2 1.4...................... 3 1.5............... 3 1.6........................... 4 1.7.................. 4
遍歴電子磁性とスピン揺らぎ理論 - 京都大学大学院理学研究科 集中講義
 email: takahash@sci.u-hyogo.ac.jp August 3, 2009 Title of Lecture: SCR Spin Fluctuation Theory 2 / 179 Part I Introduction Introduction Stoner-Wohlfarth Theory Stoner-Wohlfarth Theory Hatree Fock Approximation
email: takahash@sci.u-hyogo.ac.jp August 3, 2009 Title of Lecture: SCR Spin Fluctuation Theory 2 / 179 Part I Introduction Introduction Stoner-Wohlfarth Theory Stoner-Wohlfarth Theory Hatree Fock Approximation
(1.2) T D = 0 T = D = 30 kn 1.2 (1.4) 2F W = 0 F = W/2 = 300 kn/2 = 150 kn 1.3 (1.9) R = W 1 + W 2 = = 1100 N. (1.9) W 2 b W 1 a = 0
 1 1 1.1 1.) T D = T = D = kn 1. 1.4) F W = F = W/ = kn/ = 15 kn 1. 1.9) R = W 1 + W = 6 + 5 = 11 N. 1.9) W b W 1 a = a = W /W 1 )b = 5/6) = 5 cm 1.4 AB AC P 1, P x, y x, y y x 1.4.) P sin 6 + P 1 sin 45
1 1 1.1 1.) T D = T = D = kn 1. 1.4) F W = F = W/ = kn/ = 15 kn 1. 1.9) R = W 1 + W = 6 + 5 = 11 N. 1.9) W b W 1 a = a = W /W 1 )b = 5/6) = 5 cm 1.4 AB AC P 1, P x, y x, y y x 1.4.) P sin 6 + P 1 sin 45
(Blackbody Radiation) (Stefan-Boltzmann s Law) (Wien s Displacement Law)
 ( ) ( ) 2002.11 1 1 1.1 (Blackbody Radiation).............................. 1 1.2 (Stefan-Boltzmann s Law)................ 1 1.3 (Wien s Displacement Law)....................... 2 1.4 (Kirchhoff s Law)...........................
( ) ( ) 2002.11 1 1 1.1 (Blackbody Radiation).............................. 1 1.2 (Stefan-Boltzmann s Law)................ 1 1.3 (Wien s Displacement Law)....................... 2 1.4 (Kirchhoff s Law)...........................
( )
 7..-8..8.......................................................................... 4.................................... 3...................................... 3..3.................................. 4.3....................................
7..-8..8.......................................................................... 4.................................... 3...................................... 3..3.................................. 4.3....................................
1 No.1 5 C 1 I III F 1 F 2 F 1 F 2 2 Φ 2 (t) = Φ 1 (t) Φ 1 (t t). = Φ 1(t) t = ( 1.5e 0.5t 2.4e 4t 2e 10t ) τ < 0 t > τ Φ 2 (t) < 0 lim t Φ 2 (t) = 0
 1 No.1 5 C 1 I III F 1 F 2 F 1 F 2 2 Φ 2 (t) = Φ 1 (t) Φ 1 (t t). = Φ 1(t) t = ( 1.5e 0.5t 2.4e 4t 2e 10t ) τ < 0 t > τ Φ 2 (t) < 0 lim t Φ 2 (t) = 0 0 < t < τ I II 0 No.2 2 C x y x y > 0 x 0 x > b a dx
1 No.1 5 C 1 I III F 1 F 2 F 1 F 2 2 Φ 2 (t) = Φ 1 (t) Φ 1 (t t). = Φ 1(t) t = ( 1.5e 0.5t 2.4e 4t 2e 10t ) τ < 0 t > τ Φ 2 (t) < 0 lim t Φ 2 (t) = 0 0 < t < τ I II 0 No.2 2 C x y x y > 0 x 0 x > b a dx
卒業研究報告 題 目 Hamiltonian 指導教員 山本哲也教授 報告者 汐月康則 平成 14 年 2 月 5 日 1
 卒業研究報告 題 目 Hamiltonian 指導教員 山本哲也教授 報告者 汐月康則 平成 4 年 月 5 日 .....4.....4......6.. 6.. 6....4. 8.5. 9.6....7... 3..... 3.... 3.... 3.3...4 3.4...5 3.5...5 3.5....6 3.5.... 3.5...... 3.5...... 3 3.5.3..4 3.5.4..5
卒業研究報告 題 目 Hamiltonian 指導教員 山本哲也教授 報告者 汐月康則 平成 4 年 月 5 日 .....4.....4......6.. 6.. 6....4. 8.5. 9.6....7... 3..... 3.... 3.... 3.3...4 3.4...5 3.5...5 3.5....6 3.5.... 3.5...... 3.5...... 3 3.5.3..4 3.5.4..5
 43433 8 3 . Stochastic exponentials...................................... 3. Girsanov s theorem......................................... 4 On the martingale property of stochastic exponentials 5. Gronwall
43433 8 3 . Stochastic exponentials...................................... 3. Girsanov s theorem......................................... 4 On the martingale property of stochastic exponentials 5. Gronwall
( ) ( 40 )+( 60 ) Schrödinger 3. (a) (b) (c) yoshioka/education-09.html pdf 1
 2009 1 ( ) ( 40 )+( 60 ) 1 1. 2. Schrödinger 3. (a) (b) (c) http://goofy.phys.nara-wu.ac.jp/ yoshioka/education-09.html pdf 1 1. ( photon) ν λ = c ν (c = 3.0 108 /m : ) ɛ = hν (1) p = hν/c = h/λ (2) h
2009 1 ( ) ( 40 )+( 60 ) 1 1. 2. Schrödinger 3. (a) (b) (c) http://goofy.phys.nara-wu.ac.jp/ yoshioka/education-09.html pdf 1 1. ( photon) ν λ = c ν (c = 3.0 108 /m : ) ɛ = hν (1) p = hν/c = h/λ (2) h
: 2005 ( ρ t +dv j =0 r m m r = e E( r +e r B( r T 208 T = d E j 207 ρ t = = = e t δ( r r (t e r r δ( r r (t e r ( r δ( r r (t dv j =
 72 Maxwell. Maxwell e r ( =,,N Maxwell rot E + B t = 0 rot H D t = j dv D = ρ dv B = 0 D = ɛ 0 E H = μ 0 B ρ( r = j( r = N e δ( r r = N e r δ( r r = : 2005 ( 2006.8.22 73 207 ρ t +dv j =0 r m m r = e E(
72 Maxwell. Maxwell e r ( =,,N Maxwell rot E + B t = 0 rot H D t = j dv D = ρ dv B = 0 D = ɛ 0 E H = μ 0 B ρ( r = j( r = N e δ( r r = N e r δ( r r = : 2005 ( 2006.8.22 73 207 ρ t +dv j =0 r m m r = e E(
Part () () Γ Part ,
 Contents a 6 6 6 6 6 6 6 7 7. 8.. 8.. 8.3. 8 Part. 9. 9.. 9.. 3. 3.. 3.. 3 4. 5 4.. 5 4.. 9 4.3. 3 Part. 6 5. () 6 5.. () 7 5.. 9 5.3. Γ 3 6. 3 6.. 3 6.. 3 6.3. 33 Part 3. 34 7. 34 7.. 34 7.. 34 8. 35
Contents a 6 6 6 6 6 6 6 7 7. 8.. 8.. 8.3. 8 Part. 9. 9.. 9.. 3. 3.. 3.. 3 4. 5 4.. 5 4.. 9 4.3. 3 Part. 6 5. () 6 5.. () 7 5.. 9 5.3. Γ 3 6. 3 6.. 3 6.. 3 6.3. 33 Part 3. 34 7. 34 7.. 34 7.. 34 8. 35
 K E N Z U 2012 7 16 HP M. 1 1 4 1.1 3.......................... 4 1.2................................... 4 1.2.1..................................... 4 1.2.2.................................... 5................................
K E N Z U 2012 7 16 HP M. 1 1 4 1.1 3.......................... 4 1.2................................... 4 1.2.1..................................... 4 1.2.2.................................... 5................................
JKR Point loading of an elastic half-space 2 3 Pressure applied to a circular region Boussinesq, n =
 JKR 17 9 15 1 Point loading of an elastic half-space Pressure applied to a circular region 4.1 Boussinesq, n = 1.............................. 4. Hertz, n = 1.................................. 6 4 Hertz
JKR 17 9 15 1 Point loading of an elastic half-space Pressure applied to a circular region 4.1 Boussinesq, n = 1.............................. 4. Hertz, n = 1.................................. 6 4 Hertz
H 0 H = H 0 + V (t), V (t) = gµ B S α qb e e iωt i t Ψ(t) = [H 0 + V (t)]ψ(t) Φ(t) Ψ(t) = e ih0t Φ(t) H 0 e ih0t Φ(t) + ie ih0t t Φ(t) = [
![H 0 H = H 0 + V (t), V (t) = gµ B S α qb e e iωt i t Ψ(t) = [H 0 + V (t)]ψ(t) Φ(t) Ψ(t) = e ih0t Φ(t) H 0 e ih0t Φ(t) + ie ih0t t Φ(t) = [ H 0 H = H 0 + V (t), V (t) = gµ B S α qb e e iωt i t Ψ(t) = [H 0 + V (t)]ψ(t) Φ(t) Ψ(t) = e ih0t Φ(t) H 0 e ih0t Φ(t) + ie ih0t t Φ(t) = [](/thumbs/99/141438442.jpg) 3 3. 3.. H H = H + V (t), V (t) = gµ B α B e e iωt i t Ψ(t) = [H + V (t)]ψ(t) Φ(t) Ψ(t) = e iht Φ(t) H e iht Φ(t) + ie iht t Φ(t) = [H + V (t)]e iht Φ(t) Φ(t) i t Φ(t) = V H(t)Φ(t), V H (t) = e iht V (t)e
3 3. 3.. H H = H + V (t), V (t) = gµ B α B e e iωt i t Ψ(t) = [H + V (t)]ψ(t) Φ(t) Ψ(t) = e iht Φ(t) H e iht Φ(t) + ie iht t Φ(t) = [H + V (t)]e iht Φ(t) Φ(t) i t Φ(t) = V H(t)Φ(t), V H (t) = e iht V (t)e
 25 7 18 1 1 1.1 v.s............................. 1 1.1.1.................................. 1 1.1.2................................. 1 1.1.3.................................. 3 1.2................... 3
25 7 18 1 1 1.1 v.s............................. 1 1.1.1.................................. 1 1.1.2................................. 1 1.1.3.................................. 3 1.2................... 3
i Γ
 018 4 10 i 1 1.1.............................. 1.......................... 3 1.3............................ 6 1.4............................ 7 8.1 Γ.................................... 8.......................
018 4 10 i 1 1.1.............................. 1.......................... 3 1.3............................ 6 1.4............................ 7 8.1 Γ.................................... 8.......................
(MRI) 10. (MRI) (MRI) : (NMR) ( 1 H) MRI ρ H (x,y,z) NMR (Nuclear Magnetic Resonance) spectrometry: NMR NMR s( B ) m m = µ 0 IA = γ J (1) γ: :Planck c
 10. : (NMR) ( 1 H) MRI ρ H (x,y,z) NMR (Nuclear Magnetic Resonance) spectrometry: NMR NMR s( B ) m m = µ 0 IA = γ J (1) γ: :Planck constant J: Ĵ 2 = J(J +1),Ĵz = J J: (J = 1 2 for 1 H) I m A 173/197 10.1
10. : (NMR) ( 1 H) MRI ρ H (x,y,z) NMR (Nuclear Magnetic Resonance) spectrometry: NMR NMR s( B ) m m = µ 0 IA = γ J (1) γ: :Planck constant J: Ĵ 2 = J(J +1),Ĵz = J J: (J = 1 2 for 1 H) I m A 173/197 10.1
 1 2 27 6 12 1 HP HP @@@ 2 Quantum Physics http://as2.c.u-tokyo.ac.jp 1 1 1.1............................................ 1 1.2..................................... 1 1.3...............................................
1 2 27 6 12 1 HP HP @@@ 2 Quantum Physics http://as2.c.u-tokyo.ac.jp 1 1 1.1............................................ 1 1.2..................................... 1 1.3...............................................
総研大恒星進化概要.dvi
 The Structure and Evolution of Stars I. Basic Equations. M r r =4πr2 ρ () P r = GM rρ. r 2 (2) r: M r : P and ρ: G: M r Lagrange r = M r 4πr 2 rho ( ) P = GM r M r 4πr. 4 (2 ) s(ρ, P ) s(ρ, P ) r L r T
The Structure and Evolution of Stars I. Basic Equations. M r r =4πr2 ρ () P r = GM rρ. r 2 (2) r: M r : P and ρ: G: M r Lagrange r = M r 4πr 2 rho ( ) P = GM r M r 4πr. 4 (2 ) s(ρ, P ) s(ρ, P ) r L r T
) a + b = i + 6 b c = 6i j ) a = 0 b = c = 0 ) â = i + j 0 ˆb = 4) a b = b c = j + ) cos α = cos β = 6) a ˆb = b ĉ = 0 7) a b = 6i j b c = i + 6j + 8)
 4 4 ) a + b = i + 6 b c = 6i j ) a = 0 b = c = 0 ) â = i + j 0 ˆb = 4) a b = b c = j + ) cos α = cos β = 6) a ˆb = b ĉ = 0 7) a b = 6i j b c = i + 6j + 8) a b a b = 6i j 4 b c b c 9) a b = 4 a b) c = 7
4 4 ) a + b = i + 6 b c = 6i j ) a = 0 b = c = 0 ) â = i + j 0 ˆb = 4) a b = b c = j + ) cos α = cos β = 6) a ˆb = b ĉ = 0 7) a b = 6i j b c = i + 6j + 8) a b a b = 6i j 4 b c b c 9) a b = 4 a b) c = 7
) ] [ h m x + y + + V x) φ = Eφ 1) z E = i h t 13) x << 1) N n n= = N N + 1) 14) N n n= = N N + 1)N + 1) 6 15) N n 3 n= = 1 4 N N + 1) 16) N n 4
![) ] [ h m x + y + + V x) φ = Eφ 1) z E = i h t 13) x << 1) N n n= = N N + 1) 14) N n n= = N N + 1)N + 1) 6 15) N n 3 n= = 1 4 N N + 1) 16) N n 4 ) ] [ h m x + y + + V x) φ = Eφ 1) z E = i h t 13) x << 1) N n n= = N N + 1) 14) N n n= = N N + 1)N + 1) 6 15) N n 3 n= = 1 4 N N + 1) 16) N n 4](/thumbs/92/107866707.jpg) 1. k λ ν ω T v p v g k = π λ ω = πν = π T v p = λν = ω k v g = dω dk 1) ) 3) 4). p = hk = h λ 5) E = hν = hω 6) h = h π 7) h =6.6618 1 34 J sec) hc=197.3 MeV fm = 197.3 kev pm= 197.3 ev nm = 1.97 1 3 ev
1. k λ ν ω T v p v g k = π λ ω = πν = π T v p = λν = ω k v g = dω dk 1) ) 3) 4). p = hk = h λ 5) E = hν = hω 6) h = h π 7) h =6.6618 1 34 J sec) hc=197.3 MeV fm = 197.3 kev pm= 197.3 ev nm = 1.97 1 3 ev
ver.1 / c /(13)
 1 -- 11 1 c 2010 1/(13) 1 -- 11 -- 1 1--1 1--1--1 2009 3 t R x R n 1 ẋ = f(t, x) f = ( f 1,, f n ) f x(t) = ϕ(x 0, t) x(0) = x 0 n f f t 1--1--2 2009 3 q = (q 1,..., q m ), p = (p 1,..., p m ) x = (q,
1 -- 11 1 c 2010 1/(13) 1 -- 11 -- 1 1--1 1--1--1 2009 3 t R x R n 1 ẋ = f(t, x) f = ( f 1,, f n ) f x(t) = ϕ(x 0, t) x(0) = x 0 n f f t 1--1--2 2009 3 q = (q 1,..., q m ), p = (p 1,..., p m ) x = (q,
[ ] (Ising model) 2 i S i S i = 1 (up spin : ) = 1 (down spin : ) (4.38) s z = ±1 4 H 0 = J zn/2 i,j S i S j (4.39) i, j z 5 2 z = 4 z = 6 3
![[ ] (Ising model) 2 i S i S i = 1 (up spin : ) = 1 (down spin : ) (4.38) s z = ±1 4 H 0 = J zn/2 i,j S i S j (4.39) i, j z 5 2 z = 4 z = 6 3 [ ] (Ising model) 2 i S i S i = 1 (up spin : ) = 1 (down spin : ) (4.38) s z = ±1 4 H 0 = J zn/2 i,j S i S j (4.39) i, j z 5 2 z = 4 z = 6 3](/thumbs/103/161261933.jpg) 4.2 4.2.1 [ ] (Ising model) 2 i S i S i = 1 (up spin : ) = 1 (down spin : ) (4.38) s z = ±1 4 H 0 = J zn/2 S i S j (4.39) i, j z 5 2 z = 4 z = 6 3 z = 6 z = 8 zn/2 1 2 N i z nearest neighbors of i j=1
4.2 4.2.1 [ ] (Ising model) 2 i S i S i = 1 (up spin : ) = 1 (down spin : ) (4.38) s z = ±1 4 H 0 = J zn/2 S i S j (4.39) i, j z 5 2 z = 4 z = 6 3 z = 6 z = 8 zn/2 1 2 N i z nearest neighbors of i j=1
S I. dy fx x fx y fx + C 3 C dy fx 4 x, y dy v C xt y C v e kt k > xt yt gt [ v dt dt v e kt xt v e kt + C k x v + C C k xt v k 3 r r + dr e kt S dt d
 S I.. http://ayapin.film.s.dendai.ac.jp/~matuda /TeX/lecture.html PDF PS.................................... 3.3.................... 9.4................5.............. 3 5. Laplace................. 5....
S I.. http://ayapin.film.s.dendai.ac.jp/~matuda /TeX/lecture.html PDF PS.................................... 3.3.................... 9.4................5.............. 3 5. Laplace................. 5....
2 G(k) e ikx = (ik) n x n n! n=0 (k ) ( ) X n = ( i) n n k n G(k) k=0 F (k) ln G(k) = ln e ikx n κ n F (k) = F (k) (ik) n n= n! κ n κ n = ( i) n n k n
 . X {x, x 2, x 3,... x n } X X {, 2, 3, 4, 5, 6} X x i P i. 0 P i 2. n P i = 3. P (i ω) = i ω P i P 3 {x, x 2, x 3,... x n } ω P i = 6 X f(x) f(x) X n n f(x i )P i n x n i P i X n 2 G(k) e ikx = (ik) n
. X {x, x 2, x 3,... x n } X X {, 2, 3, 4, 5, 6} X x i P i. 0 P i 2. n P i = 3. P (i ω) = i ω P i P 3 {x, x 2, x 3,... x n } ω P i = 6 X f(x) f(x) X n n f(x i )P i n x n i P i X n 2 G(k) e ikx = (ik) n
, 1.,,,.,., (Lin, 1955).,.,.,.,. f, 2,. main.tex 2011/08/13( )
 81 4 2 4.1, 1.,,,.,., (Lin, 1955).,.,.,.,. f, 2,. 82 4.2. ζ t + V (ζ + βy) = 0 (4.2.1), V = 0 (4.2.2). (4.2.1), (3.3.66) R 1 Φ / Z, Γ., F 1 ( 3.2 ). 7,., ( )., (4.2.1) 500 hpa., 500 hpa (4.2.1) 1949,.,
81 4 2 4.1, 1.,,,.,., (Lin, 1955).,.,.,.,. f, 2,. 82 4.2. ζ t + V (ζ + βy) = 0 (4.2.1), V = 0 (4.2.2). (4.2.1), (3.3.66) R 1 Φ / Z, Γ., F 1 ( 3.2 ). 7,., ( )., (4.2.1) 500 hpa., 500 hpa (4.2.1) 1949,.,
1. (8) (1) (x + y) + (x + y) = 0 () (x + y ) 5xy = 0 (3) (x y + 3y 3 ) (x 3 + xy ) = 0 (4) x tan y x y + x = 0 (5) x = y + x + y (6) = x + y 1 x y 3 (
 1 1.1 (1) (1 + x) + (1 + y) = 0 () x + y = 0 (3) xy = x (4) x(y + 3) + y(y + 3) = 0 (5) (a + y ) = x ax a (6) x y 1 + y x 1 = 0 (7) cos x + sin x cos y = 0 (8) = tan y tan x (9) = (y 1) tan x (10) (1 +
1 1.1 (1) (1 + x) + (1 + y) = 0 () x + y = 0 (3) xy = x (4) x(y + 3) + y(y + 3) = 0 (5) (a + y ) = x ax a (6) x y 1 + y x 1 = 0 (7) cos x + sin x cos y = 0 (8) = tan y tan x (9) = (y 1) tan x (10) (1 +
18 I ( ) (1) I-1,I-2,I-3 (2) (3) I-1 ( ) (100 ) θ ϕ θ ϕ m m l l θ ϕ θ ϕ 2 g (1) (2) 0 (3) θ ϕ (4) (3) θ(t) = A 1 cos(ω 1 t + α 1 ) + A 2 cos(ω 2 t + α
 18 I ( ) (1) I-1,I-2,I-3 (2) (3) I-1 ( ) (100 ) θ ϕ θ ϕ m m l l θ ϕ θ ϕ 2 g (1) (2) 0 (3) θ ϕ (4) (3) θ(t) = A 1 cos(ω 1 t + α 1 ) + A 2 cos(ω 2 t + α 2 ), ϕ(t) = B 1 cos(ω 1 t + α 1 ) + B 2 cos(ω 2 t
18 I ( ) (1) I-1,I-2,I-3 (2) (3) I-1 ( ) (100 ) θ ϕ θ ϕ m m l l θ ϕ θ ϕ 2 g (1) (2) 0 (3) θ ϕ (4) (3) θ(t) = A 1 cos(ω 1 t + α 1 ) + A 2 cos(ω 2 t + α 2 ), ϕ(t) = B 1 cos(ω 1 t + α 1 ) + B 2 cos(ω 2 t
untitled
 18 1 2,000,000 2,000,000 2007 2 2 2008 3 31 (1) 6 JCOSSAR 2007pp.57-642007.6. LCC (1) (2) 2 10mm 1020 14 12 10 8 6 4 40,50,60 2 0 1998 27.5 1995 1960 40 1) 2) 3) LCC LCC LCC 1 1) Vol.42No.5pp.29-322004.5.
18 1 2,000,000 2,000,000 2007 2 2 2008 3 31 (1) 6 JCOSSAR 2007pp.57-642007.6. LCC (1) (2) 2 10mm 1020 14 12 10 8 6 4 40,50,60 2 0 1998 27.5 1995 1960 40 1) 2) 3) LCC LCC LCC 1 1) Vol.42No.5pp.29-322004.5.
 2007 5 iii 1 1 1.1.................... 1 2 5 2.1 (shear stress) (shear strain)...... 5 2.1.1...................... 6 2.1.2.................... 6 2.2....................... 7 2.2.1........................
2007 5 iii 1 1 1.1.................... 1 2 5 2.1 (shear stress) (shear strain)...... 5 2.1.1...................... 6 2.1.2.................... 6 2.2....................... 7 2.2.1........................
液晶の物理1:連続体理論(弾性,粘性)
 The Physics of Liquid Crystals P. G. de Gennes and J. Prost (Oxford University Press, 1993) Liquid crystals are beautiful and mysterious; I am fond of them for both reasons. My hope is that some readers
The Physics of Liquid Crystals P. G. de Gennes and J. Prost (Oxford University Press, 1993) Liquid crystals are beautiful and mysterious; I am fond of them for both reasons. My hope is that some readers
Riemann-Stieltjes Poland S. Lojasiewicz [1] An introduction to the theory of real functions, John Wiley & Sons, Ltd., Chichester, 1988.,,,,. Riemann-S
![Riemann-Stieltjes Poland S. Lojasiewicz [1] An introduction to the theory of real functions, John Wiley & Sons, Ltd., Chichester, 1988.,,,,. Riemann-S Riemann-Stieltjes Poland S. Lojasiewicz [1] An introduction to the theory of real functions, John Wiley & Sons, Ltd., Chichester, 1988.,,,,. Riemann-S](/thumbs/94/118569863.jpg) Riemnn-Stieltjes Polnd S. Lojsiewicz [1] An introduction to the theory of rel functions, John Wiley & Sons, Ltd., Chichester, 1988.,,,, Riemnn-Stieltjes 1 2 2 5 3 6 4 Jordn 13 5 Riemnn-Stieltjes 15 6 Riemnn-Stieltjes
Riemnn-Stieltjes Polnd S. Lojsiewicz [1] An introduction to the theory of rel functions, John Wiley & Sons, Ltd., Chichester, 1988.,,,, Riemnn-Stieltjes 1 2 2 5 3 6 4 Jordn 13 5 Riemnn-Stieltjes 15 6 Riemnn-Stieltjes
i
 009 I 1 8 5 i 0 1 0.1..................................... 1 0.................................................. 1 0.3................................. 0.4........................................... 3
009 I 1 8 5 i 0 1 0.1..................................... 1 0.................................................. 1 0.3................................. 0.4........................................... 3
1. z dr er r sinθ dϕ eϕ r dθ eθ dr θ dr dθ r x 0 ϕ r sinθ dϕ r sinθ dϕ y dr dr er r dθ eθ r sinθ dϕ eϕ 2. (r, θ, φ) 2 dr 1 h r dr 1 e r h θ dθ 1 e θ h
 IB IIA 1 1 r, θ, φ 1 (r, θ, φ)., r, θ, φ 0 r
IB IIA 1 1 r, θ, φ 1 (r, θ, φ)., r, θ, φ 0 r
6 6.1 B A: Γ d Q S(B) S(A) = S (6.1) T (e) Γ (6.2) : Γ B A R (reversible) 6-1
 6 6.1 B A: Γ d Q S(B) S(A) = S (6.1) (e) Γ (6.2) : Γ B A R (reversible) 6-1 (e) = Clausius 0 = B A: Γ B A: Γ d Q A + d Q (e) B: R d Q + S(A) S(B) (6.3) (e) // 6.2 B A: Γ d Q S(B) S(A) = S (6.4) (e) Γ (6.5)
6 6.1 B A: Γ d Q S(B) S(A) = S (6.1) (e) Γ (6.2) : Γ B A R (reversible) 6-1 (e) = Clausius 0 = B A: Γ B A: Γ d Q A + d Q (e) B: R d Q + S(A) S(B) (6.3) (e) // 6.2 B A: Γ d Q S(B) S(A) = S (6.4) (e) Γ (6.5)
1 2 2 (Dielecrics) Maxwell ( ) D H
 2003.02.13 1 2 2 (Dielecrics) 4 2.1... 4 2.2... 5 2.3... 6 2.4... 6 3 Maxwell ( ) 9 3.1... 9 3.2 D H... 11 3.3... 13 4 14 4.1... 14 4.2... 14 4.3... 17 4.4... 19 5 22 6 THz 24 6.1... 24 6.2... 25 7 26
2003.02.13 1 2 2 (Dielecrics) 4 2.1... 4 2.2... 5 2.3... 6 2.4... 6 3 Maxwell ( ) 9 3.1... 9 3.2 D H... 11 3.3... 13 4 14 4.1... 14 4.2... 14 4.3... 17 4.4... 19 5 22 6 THz 24 6.1... 24 6.2... 25 7 26
untitled
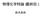 D nucleation 3 3D nucleation Glucose isomerase 10 V / nm s -1 5 0 0 5 10 C - C e / mg ml -1 kinetics µ R K kt kinetics kinetics kinetics r β π µ π r a r s + a s : β: µ πβ µ β s c s c a a r, & exp exp
D nucleation 3 3D nucleation Glucose isomerase 10 V / nm s -1 5 0 0 5 10 C - C e / mg ml -1 kinetics µ R K kt kinetics kinetics kinetics r β π µ π r a r s + a s : β: µ πβ µ β s c s c a a r, & exp exp
,,,17,,, ( ),, E Q [S T F t ] < S t, t [, T ],,,,,,,,
![,,,17,,, ( ),, E Q [S T F t ] < S t, t [, T ],,,,,,,, ,,,17,,, ( ),, E Q [S T F t ] < S t, t [, T ],,,,,,,,](/thumbs/91/105754403.jpg) 14 5 1 ,,,17,,,194 1 4 ( ),, E Q [S T F t ] < S t, t [, T ],,,,,,,, 1 4 1.1........................................ 4 5.1........................................ 5.........................................
14 5 1 ,,,17,,,194 1 4 ( ),, E Q [S T F t ] < S t, t [, T ],,,,,,,, 1 4 1.1........................................ 4 5.1........................................ 5.........................................
現代物理化学 1-1(4)16.ppt
 (pdf) pdf pdf http://www1.doshisha.ac.jp/~bukka/lecture/index.html http://www.doshisha.ac.jp/ Duet -1-1-1 2-a. 1-1-2 EU E = K E + P E + U ΔE K E = 0P E ΔE = ΔU U U = εn ΔU ΔU = Q + W, du = d 'Q + d 'W
(pdf) pdf pdf http://www1.doshisha.ac.jp/~bukka/lecture/index.html http://www.doshisha.ac.jp/ Duet -1-1-1 2-a. 1-1-2 EU E = K E + P E + U ΔE K E = 0P E ΔE = ΔU U U = εn ΔU ΔU = Q + W, du = d 'Q + d 'W
(2 X Poisso P (λ ϕ X (t = E[e itx ] = k= itk λk e k! e λ = (e it λ k e λ = e eitλ e λ = e λ(eit 1. k! k= 6.7 X N(, 1 ϕ X (t = e 1 2 t2 : Cauchy ϕ X (t
![(2 X Poisso P (λ ϕ X (t = E[e itx ] = k= itk λk e k! e λ = (e it λ k e λ = e eitλ e λ = e λ(eit 1. k! k= 6.7 X N(, 1 ϕ X (t = e 1 2 t2 : Cauchy ϕ X (t (2 X Poisso P (λ ϕ X (t = E[e itx ] = k= itk λk e k! e λ = (e it λ k e λ = e eitλ e λ = e λ(eit 1. k! k= 6.7 X N(, 1 ϕ X (t = e 1 2 t2 : Cauchy ϕ X (t](/thumbs/67/56612994.jpg) 6 6.1 6.1 (1 Z ( X = e Z, Y = Im Z ( Z = X + iy, i = 1 (2 Z E[ e Z ] < E[ Im Z ] < Z E[Z] = E[e Z] + ie[im Z] 6.2 Z E[Z] E[ Z ] : E[ Z ] < e Z Z, Im Z Z E[Z] α = E[Z], Z = Z Z 1 {Z } E[Z] = α = α [ α ]
6 6.1 6.1 (1 Z ( X = e Z, Y = Im Z ( Z = X + iy, i = 1 (2 Z E[ e Z ] < E[ Im Z ] < Z E[Z] = E[e Z] + ie[im Z] 6.2 Z E[Z] E[ Z ] : E[ Z ] < e Z Z, Im Z Z E[Z] α = E[Z], Z = Z Z 1 {Z } E[Z] = α = α [ α ]
<4D F736F F D B B83578B6594BB2D834A836F815B82D082C88C60202E646F63>
 基礎からの冷凍空調 サンプルページ この本の定価 判型などは, 以下の URL からご覧いただけます. http://www.morikita.co.jp/books/mid/067311 このサンプルページの内容は, 初版 1 刷発行当時のものです. http://www.morikita.co.jp/support. 03-3817-5670FAX 03-3815-8199 i () () Q&A
基礎からの冷凍空調 サンプルページ この本の定価 判型などは, 以下の URL からご覧いただけます. http://www.morikita.co.jp/books/mid/067311 このサンプルページの内容は, 初版 1 刷発行当時のものです. http://www.morikita.co.jp/support. 03-3817-5670FAX 03-3815-8199 i () () Q&A
SFGÇÃÉXÉyÉNÉgÉãå`.pdf
 SFG 1 SFG SFG I SFG (ω) χ SFG (ω). SFG χ χ SFG (ω) = χ NR e iϕ +. ω ω + iγ SFG φ = ±π/, χ φ = ±π 3 χ SFG χ SFG = χ NR + χ (ω ω ) + Γ + χ NR χ (ω ω ) (ω ω ) + Γ cosϕ χ NR χ Γ (ω ω ) + Γ sinϕ. 3 (θ) 180
SFG 1 SFG SFG I SFG (ω) χ SFG (ω). SFG χ χ SFG (ω) = χ NR e iϕ +. ω ω + iγ SFG φ = ±π/, χ φ = ±π 3 χ SFG χ SFG = χ NR + χ (ω ω ) + Γ + χ NR χ (ω ω ) (ω ω ) + Γ cosϕ χ NR χ Γ (ω ω ) + Γ sinϕ. 3 (θ) 180
S I. dy fx x fx y fx + C 3 C vt dy fx 4 x, y dy yt gt + Ct + C dt v e kt xt v e kt + C k x v k + C C xt v k 3 r r + dr e kt S Sr πr dt d v } dt k e kt
 S I. x yx y y, y,. F x, y, y, y,, y n http://ayapin.film.s.dendai.ac.jp/~matuda n /TeX/lecture.html PDF PS yx.................................... 3.3.................... 9.4................5..............
S I. x yx y y, y,. F x, y, y, y,, y n http://ayapin.film.s.dendai.ac.jp/~matuda n /TeX/lecture.html PDF PS yx.................................... 3.3.................... 9.4................5..............
gr09.dvi
 .1, θ, ϕ d = A, t dt + B, t dtd + C, t d + D, t dθ +in θdϕ.1.1 t { = f1,t t = f,t { D, t = B, t =.1. t A, tdt e φ,t dt, C, td e λ,t d.1.3,t, t d = e φ,t dt + e λ,t d + dθ +in θdϕ.1.4 { = f1,t t = f,t {
.1, θ, ϕ d = A, t dt + B, t dtd + C, t d + D, t dθ +in θdϕ.1.1 t { = f1,t t = f,t { D, t = B, t =.1. t A, tdt e φ,t dt, C, td e λ,t d.1.3,t, t d = e φ,t dt + e λ,t d + dθ +in θdϕ.1.4 { = f1,t t = f,t {
 006 11 8 0 3 1 5 1.1..................... 5 1......................... 6 1.3.................... 6 1.4.................. 8 1.5................... 8 1.6................... 10 1.6.1......................
006 11 8 0 3 1 5 1.1..................... 5 1......................... 6 1.3.................... 6 1.4.................. 8 1.5................... 8 1.6................... 10 1.6.1......................
1 filename=mathformula tex 1 ax 2 + bx + c = 0, x = b ± b 2 4ac, (1.1) 2a x 1 + x 2 = b a, x 1x 2 = c a, (1.2) ax 2 + 2b x + c = 0, x = b ± b 2
 filename=mathformula58.tex ax + bx + c =, x = b ± b 4ac, (.) a x + x = b a, x x = c a, (.) ax + b x + c =, x = b ± b ac. a (.3). sin(a ± B) = sin A cos B ± cos A sin B, (.) cos(a ± B) = cos A cos B sin
filename=mathformula58.tex ax + bx + c =, x = b ± b 4ac, (.) a x + x = b a, x x = c a, (.) ax + b x + c =, x = b ± b ac. a (.3). sin(a ± B) = sin A cos B ± cos A sin B, (.) cos(a ± B) = cos A cos B sin
(3) (2),,. ( 20) ( s200103) 0.7 x C,, x 2 + y 2 + ax = 0 a.. D,. D, y C, C (x, y) (y 0) C m. (2) D y = y(x) (x ± y 0), (x, y) D, m, m = 1., D. (x 2 y
 [ ] 7 0.1 2 2 + y = t sin t IC ( 9) ( s090101) 0.2 y = d2 y 2, y = x 3 y + y 2 = 0 (2) y + 2y 3y = e 2x 0.3 1 ( y ) = f x C u = y x ( 15) ( s150102) [ ] y/x du x = Cexp f(u) u (2) x y = xey/x ( 16) ( s160101)
[ ] 7 0.1 2 2 + y = t sin t IC ( 9) ( s090101) 0.2 y = d2 y 2, y = x 3 y + y 2 = 0 (2) y + 2y 3y = e 2x 0.3 1 ( y ) = f x C u = y x ( 15) ( s150102) [ ] y/x du x = Cexp f(u) u (2) x y = xey/x ( 16) ( s160101)
輻射の量子論、選択則、禁制線、許容線
 Radiative Processes in Astrophysics 005/8/1 http://wwwxray.ess.sci.osaka- u.ac.jp/~hayasida Semi-Classical Theory of Radiative Transitions r r 1/ 4 H = ( cp ea) m c + + eφ nonrelativistic limit, Coulomb
Radiative Processes in Astrophysics 005/8/1 http://wwwxray.ess.sci.osaka- u.ac.jp/~hayasida Semi-Classical Theory of Radiative Transitions r r 1/ 4 H = ( cp ea) m c + + eφ nonrelativistic limit, Coulomb
ADM-Hamiltonian Cheeger-Gromov 3. Penrose
 ADM-Hamiltonian 1. 2. Cheeger-Gromov 3. Penrose 0. ADM-Hamiltonian (M 4, h) Einstein-Hilbert M 4 R h hdx L h = R h h δl h = 0 (Ric h ) αβ 1 2 R hg αβ = 0 (Σ 3, g ij ) (M 4, h ij ) g ij, k ij Σ π ij = g(k
ADM-Hamiltonian 1. 2. Cheeger-Gromov 3. Penrose 0. ADM-Hamiltonian (M 4, h) Einstein-Hilbert M 4 R h hdx L h = R h h δl h = 0 (Ric h ) αβ 1 2 R hg αβ = 0 (Σ 3, g ij ) (M 4, h ij ) g ij, k ij Σ π ij = g(k
1 variation 1.1 imension unit L m M kg T s Q C QT 1 A = C s 1 MKSA F = ma N N = kg m s 1.1 J E = 1 mv W = F x J = kg m s 1 = N m 1.
 1.1 1. 1.3.1..3.4 3.1 3. 3.3 4.1 4. 4.3 5.1 5. 5.3 6.1 6. 6.3 7.1 7. 7.3 1 1 variation 1.1 imension unit L m M kg T s Q C QT 1 A = C s 1 MKSA F = ma N N = kg m s 1.1 J E = 1 mv W = F x J = kg m s 1 = N
1.1 1. 1.3.1..3.4 3.1 3. 3.3 4.1 4. 4.3 5.1 5. 5.3 6.1 6. 6.3 7.1 7. 7.3 1 1 variation 1.1 imension unit L m M kg T s Q C QT 1 A = C s 1 MKSA F = ma N N = kg m s 1.1 J E = 1 mv W = F x J = kg m s 1 = N
QMII_10.dvi
 65 1 1.1 1.1.1 1.1 H H () = E (), (1.1) H ν () = E ν () ν (). (1.) () () = δ, (1.3) μ () ν () = δ(μ ν). (1.4) E E ν () E () H 1.1: H α(t) = c (t) () + dνc ν (t) ν (), (1.5) H () () + dν ν () ν () = 1 (1.6)
65 1 1.1 1.1.1 1.1 H H () = E (), (1.1) H ν () = E ν () ν (). (1.) () () = δ, (1.3) μ () ν () = δ(μ ν). (1.4) E E ν () E () H 1.1: H α(t) = c (t) () + dνc ν (t) ν (), (1.5) H () () + dν ν () ν () = 1 (1.6)
構造と連続体の力学基礎
 II 37 Wabash Avenue Bridge, Illinois 州 Winnipeg にある歩道橋 Esplanade Riel 橋6 6 斜張橋である必要は多分無いと思われる すぐ横に道路用桁橋有り しかも塔基部のレストランは 8 年には営業していなかった 9 9. 9.. () 97 [3] [5] k 9. m w(t) f (t) = f (t) + mg k w(t) Newton
II 37 Wabash Avenue Bridge, Illinois 州 Winnipeg にある歩道橋 Esplanade Riel 橋6 6 斜張橋である必要は多分無いと思われる すぐ横に道路用桁橋有り しかも塔基部のレストランは 8 年には営業していなかった 9 9. 9.. () 97 [3] [5] k 9. m w(t) f (t) = f (t) + mg k w(t) Newton
(e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ,µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R,µ R,τ R (2.1a
![(e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ,µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R,µ R,τ R (2.1a (e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ,µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R,µ R,τ R (2.1a](/thumbs/100/144882653.jpg) 1 2 2.1 (e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ,µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R,µ R,τ R (2.1a) L ( ) ) * 2) W Z 1/2 ( - ) d u + e + ν e 1 1 0 0
1 2 2.1 (e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ,µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R,µ R,τ R (2.1a) L ( ) ) * 2) W Z 1/2 ( - ) d u + e + ν e 1 1 0 0
