日本化学療法学会雑誌第54巻第S-1号
|
|
|
- あきお にかどり
- 5 years ago
- Views:
Transcription
1 Key words β β
2 Table. CmaxandAUCinsingleintravenousinjectionstudy ITCZAdministration (mg) n Cmax(ng/mL) ITCZ OH-ITCZ AUC( )(ng hr/ml) ITCZ OH-ITCZ 5 7.7± ±.,959.7±.9,78.± 557.,59.±.9.± 5. 5,8.7± 97.,77.±,7.,.7±, ±. 7,.±,79.,.5±9,. Mean±S.D. [JanssenPharmaceuticalInternalData] Table. CmaxandAUCinrepeatedintravenousinjectionstudy ITCZAdministration (mg) n Cmax(ng/mL) ITCZ OH-ITCZ AUC( )(ng hr/ml) ITCZ OH-ITCZ,98.±.7,.±8. 5,.±,598.,98.5±,97.5,57.7±58.9,8.5±58.9,8.5±7,8.,9.±9,85. Mean±S.D. [JanssenPharmaceuticalInternalData] I
3 StudyPeriod ITCZInjectiontreatment Priorto Injections Examination andobservation ITCZInjection ClinicalManifestation FungalTest SerologicTestforFungi GeneticTestforFungi EndoscopicExamination DiagnosticImaging (X-ray,CTscan,etc) HematologicalTest/ BloodBiochemicalTest UrineTest hrcreatinineclearance Test Cardiogram AdverseEvent Blood Sample to Testfor AmountofMedication in Plasma StudyPeriod Examination andobservation ITCZCapsule Administration Table. Examination/observationandimplementation Day Day Day Day Day Injection Day Week Week Week Week Twice/dayTwice/day 滑害害害害害 Once/day 害害害害害葛 滑害害害害 SpecimenColectionPeriod 害害害害葛 滑害害害害害害害 Twice 害害害害害害害葛 滑害害害害害害害害害 Asnecessary 害害害害害害害害害害葛 Day5 Initial Day 滑害害害害 AccordingtoSymptoms 害害害害葛 滑害害害害 AccordingtoSymptoms 害害害害葛 滑害害害害害害害害害害害害害害害害害害害害害害害害葛 Week Week Week Week Week Week8 CapsuleAdministration Week Week Week Week8 Week Week Week Week ITCZCapsuleAdministration 滑害害害害害害害害害害害害害害害害 Twice/day 害害害害害害害害害害害害害害害害害葛 ClinicalManifestation FungalTest SerologicTestforFungi EndoscopicExamination DiagnosticImaging (X-ray,CTscan,etc) HematologicalTest/ BloodBiochemicalTest UrineTest Cardiogram AdverseEvent Blood Sample to Testfor AmountofMedication in Plasma 滑害害害害害害害害害害害 SpecimenColectionPeriod 害害害害害害害害害害害葛 滑害害害害害害害害害害害害 AccordingtoSymptoms 害害害害害害害害害害害害葛 滑害害害害害害害害害害害害 AccordingtoSymptoms 害害害害害害害害害害害害葛 Days AfterFinal Administration 滑害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害害葛 required HematologicalTests:WBCcount,RBCcount,hemoglobin,hematocrit,plateletcount,diferentialcountofleukocytes BloodBiochemicalTests:totalbilirubin,ALP,AST(GOT),ALT(GPT),LDH,γ-GTP,totalprotein,albumin,glucose,totalcholesterol,triglyceride,CK,BUN,creatinine,ureanitrogen,Ca,Na,K,Cl,CRP UrineTests:sugar,protein,urobilinogen,sediment
4 Table. Totalefectcriteria TotalEfect(DeepMycosisexcludingAspergilomaandAsymptomaticPulmonaryCryptococcosis) Efective:Whenclinicalsymptomsofdeepmycosisdisappear/improve,andnoresultsfrom mycologicandserologictests,orendoscopyand diagnosticimaging(x-ray,ct,etc.),indicateincreasing/worsening,andatleastoneofthefolowingpointsapply: )Mycologicaltestsevaluatefungusasdisappeared/presumeddisappearedorreduced. )Fungalserologicaltestsreturnnegativeorimprovedresults. )Thedegreeofimprovementassessedbyendoscopyanddiagnosticimagingindicatesymptomshavedisappearedorimproved. NoEfect:Whentotalefectsarecategorizedneitherasefectivenorimpossibletoevaluate. ImpossibletoEvaluate:Whentheimprovementofclinicalsymptomsisassessedasimpossibletoevaluate,orclinicalsymptomsfordeepmycosisaredeterminedtohavedisappeared/improved,butfungalserologicaltestsandendoscopyanddiagnosticimagingalindicate thatimprovementassessmentisimpossible. TotalEfect(Aspergiloma) Efective:Whenclinicalsymptomsofaspergilomadisappear/improve,andnoresultsfrom mycologicalandserologicaltests,orendoscopy anddiagnosticimaging(x-ray,ct,etc.),indicateincreasing/worsening,andatleastoneofthefolowingpointsapply. )Thedegreeofimprovementassessedbydiagnosticimagingindicatesymptomshavedisappearedorimproved. )Mycologicaltestsevaluatefungusasdisappeared/presumeddisappeared. NoEfect:Whentotalefectsarecategorizedneitherasefectivenorimpossibletoevaluate. ImpossibletoEvaluate:Whenimprovementofclinicalsymptomsisassessedasimpossibletoevaluate,orclinicalsymptomsforaspergiloma aredeterminedtohavedisappeared/improved,butthedegreeofimprovementevaluatedbyimagingisunchangedorisimpossible toevaluate,themycologictestsindicatedecreased/unchangedorimpossibletoevaluate,andfungalserologicaltestsarenotassessedasworsening. TotalEfect(AsymptomaticPulmonaryCryptococcosis) Efective:Whenresultsfrom mycologicalandserologicaltests,andendoscopyanddiagnosticimaging(x-ray,ct,etc.)donotindicate increasing/worsening,andatleastoneofthefolowingpointsapply: )Mycologicaltestsevaluatefungusasdisappeared/presumeddisappearedorreduced. )Fungalserologicaltestsreturnnegativeorimprovedresults. )Thedegreeofimprovementassessedbyendoscopyanddiagnosticimagingindicatesymptomshavedisappearedorimproved. NoEfect:Whentotalefectsarecategorizedneitherasefectivenorimpossibletoevaluate. ImpossibletoEvaluate:Whentheimprovementofclinicalsymptoms,mycologicaltestresults,fungalserologicaltestresults,andendoscopy anddiagnosticimagingresultsarealassessedasimpossibletoevaluate. ITCZ Injection: 5 Subjects Safety analysis population: 5 Subjects Excluded from efficacy analysis population: Subjects Incompatible with basic assessment Impossible to evaluate total effects 7 Exclusion criteria/violation of concomitant medication Poststudy disqualification ) Insufficient administration ) After the study had begun, an unanticipated conflict became apparent, and administration was discontinued. Efficacy analysis population: Subjects ) Aspergillosis 9 Candidiasis 7 Cryptococcosis 5 ) Some subjects presented with both pulmonary cryptococcosis and esophageal candidiasis, and were included in both groups. Fig.. Numberofsubjectsforanalysis.
5 Gender Age(years) Weight(kg) Diagnosis Underlying Medical Condition Useof antifungal medication startedwithin weeks priortoinitial administration ofstudy medication Male Female Disease Subjects Mean±S.D. (Min,Max) < 5 5 Mean±S.D. (Min,Max) ChronicNecrotizingPulmonary Aspergilosis InvasivePulmonaryAspergilosis Aspergiloma AspergiloticOsteomyelitis AspergilusBrainAbscess Candidemia DisseminatedCandidiasis PulmonaryCandidiasis EsophagealCandidiasis UrinaryCandidiasis PulmonaryCryptococcosis Table5. Patientprofile Aspergilosis ±.9 (,79) 7 9.±9.7 (.8,9.) 9 Candidiasis 57.±.5 (,8) 5.±. (7.5,9.) Cryptococcosis.5±. (,77) 5.±. (.5,7.5) Deep Mycosis ) ±5.8 (9,79) 5.9±9.9 (.7,9.8) Total ±5.7 (9,79) 5 5.9±. (.8,7.5) DeepMycosis ) None Existing 5 Main Disease ) None Existing Blood/Hematopoietic Respiratory Colagen SolidMalignantTumor ObsoletePulmonaryTB Diabetes 7 8 Fluconazole Medication ) AmphotericinB 7 5 Miconazole Reasonfor InsuficientEfects 9 change ) SideEfects ) Deepmycosisthatdoesnotfulfildiagnosticcriteria, ) Includesduplications II
6 Table. Eficacy Disease Diagnosis Aspergilosis InvasivePulmonaryAspergilosis ChronicNecronicPulmonaryAspergilosis Aspergiloma AspergiloticOsteomyelitis Candidiasis Candidemia DisseminatedCandidiasis PulmonaryCandidiasis EsophagealCandidiasis UrinaryCandidiasis Cryptococcosis PulmonaryCryptococcosis Total E/SE * ITCZInjection Evaluation 7/7 / 5/8 / / /7 / / / / / /5 /5 5/9 ER ** E/SE * /9 / 5/8 / / 5/7 / / / / / 5/5 5/5 / * E/SE:Eficacy/SubjectsEvaluated, ** ER:EficacyRate, *** CI:ConfidenceInterval FinalEvaluation ER ** 95%CI *** 57.9 [ ] 5. [.8-9.].5 [.5-9.5] 5. [.8-88.] [.5-] 7. [9-9.] 5. [.-98.7] [ -97.5] [.5-] [5.8-] [.5-] [7.8-] [7.8-] 7.7 [8.-8.] Disease Diagnosis Aspergilosis InvasivePulmonaryAspergilosis ChronicNecronicPulmonaryAspergilosis Aspergiloma AspergiloticOsteomyelitis Candidiasis Candidemia DisseminatedCandidiasis PulmonaryCandidiasis EsophagealCandidiasis UrinaryCandidiasis Cryptococcosis PulmonaryCryptococcosis Total Table7. Itemizedevaluationresultsfrom finalevaluation Improvementof ClinicalSymptoms IR ) /9 / /8 / / 7/7 / / / / / / / / Mycological Results DR ) /7 / / / / / / / / / / / Fungal Serological Results IR ) 9/8 / /7 / / / / / / / / / / Improvementof Endoscopyand Imaging ) Improvement=(numberofsubjectswithdisappearance+ numberofsubjectswithimprovementofsymptoms)/(subjectsevaluated,ex cludingsubjectsimpossibletoevaluate) ) Disappearance=(numberofsubjectswithdisappearanceorassumeddisappearance)/(subjectsevaluated,excludingsubjectsimpossible toevaluate) ) Improvement=(numberofsubjectswithanegativeresult+ numberofsubjectswithanimprovedresult)/(subjectsevaluated,excluding subjectsimpossibletoevaluate) IR ) 8/9 / /8 / / / / / / 5/5 5/5 /
7 Table8. Eficacyforspecificfungusinfinalevaluation UnderlyingFungus Aspergilusfumigatus Aspergilustereus E/SE * /7 / ER ** %CI *** [8.-9.] [.5-] Candidaalbicans ) /5. [.7-9.7] Candidaglabrata ) / [5.8-] Candidatropicalis / [.5-] Cryptococcusneoformans 5/5 [7.8-] * E/SE:Eficacy/SubjectsEvaluated, ** ER:EficacyRate, *** CI:ConfidenceInterval ) OnesubjectwhohadCandidaalbicansandCandidaglabratais includedinbothresults. Table9. Eficacyformaindiseases Disease Underlying Circulatory Respiratory Colagen SolidMalignantTumor Obsolete Pulmonary Tuberculosis Diabetes ER E/SE * ** /5 / / / /5 / Aspergilosis 95%CI ***. [.7-9.7].7 [.-95.7] [.5- ] [.5- ]. [ ].7 [ ] ER E/SE * ** / / / Candidiasis 95%CI ***.7 [ ] [.5- ] 5. [.-98.7] ER E/SE * ** / / / * E/SE:Eficacy/SubjectsEvaluated, ** ER:EficacyRate, *** CI:ConfidenceInterval Cryptococcosis 95%CI *** [.5- ] [5.8- ] [.5- ] E/SE * 5/8 5/7 / / /5 / ER ** Total 95%CI *** [.5-9.5] [9.-9.] [9.8- ] [ ] [ ] [9.-99.] % 8 Impossible to evaluate Worsened Unchanged Clinical improvement Improved Disappeared Pre 8 W ITCZ Injection ITCZ Capsule Administration n Fig.. Improvementinclinicalsymptoms.
8 Body Temperature C Mean S.D. t-test vs. Preadministration p.5 p.. Pre D W ITCZ Injection W ITCZ Capsule Administration 8 W n mg/dl 8 Mean S.D. t-test vs. Preadministration p.5 p. CRP Pre 8 W D W W ITCZ Injection ITCZ Capsule Administration n Fig.. ChangeinbodytemperatureandCRP. β β β
9 ng/ml, Plasma Concentration of Medication 5,,,,, ITCZ OH-ITCZ Mean S.D. Pre W D W W ITCZ Injection ITCZ Capsule Administration n Fig.. Changeinplasmaconcentrationofmedication(subjectsreceivingmgITCZ/injection). ng/ml, Plasma Concentration of Medication 5,,,,, ITCZ mg/injection, mg/capsule ITCZ mg/injection, mg/capsule OH-ITCZ mg/injection, mg/capsule OH-ITCZ mg/injection, mg/capsule Mean±S.D. Pre D W W ITCZ Injection ITCZ Capsule Administration mg/ capsule n mg/ n capsule Fig.5. Changeinplasmaconcentrationofmedication(subjectsweighinglessthan5kgwhoreceived mgofitczinjection). W
10 Table. MICvalueforITCZclassifiedbyunderlyingfungus IsolatedFungus Aspergilusfumigatus Aspergilusniger Aspergilustereus Candidaglabrata Candidaalbicans Candidatropicalis Cryptococcusneoformans Cases 5 5 MIC(μ g/ml) Min Max orless..5.5.orless. III
11 Table. Occurenceofadvancereactionclassifiedbygrade Grade Severe Moderate Mild AdverseEvent System organclass Cases Cases Cases PneumoniaNOS InfectionandGiardiasis. Thrombocytopenia BloodorLymphDisorder AnemiaNOS. Hyperkalemia Metabolicor NutrientDisorder. Hypertriglyceridemia. LossofAppetiteNOS 7.8 Anorexia..7 7 Hypokalemia. Anxiety MentalDisorder. DepressionSymptoms. LossofSensation NervousSystem Disorder. Drowsiness. Tremor Headache. Dysphonia 5.9 Dizziness. AbnormalPalate. RetinalHemorhage OcularDisorder. CongestiveHeartFailure HeartDisorder. RightBundleBranchBlock. BradycardiaNOS HeartFailureNOS Palpitation. TachycardiaNOS. ValvularCardiacDiseaseNOS.. AngiopathyNOS CirculatoryDisorder. Erubescence. Coughing Respiratory,Thoracic, ormediastinaldisorder. Dyspnea. LungHemorhage Nausea GastrointestinalDisorder. GastritisNOS. GastroduodenalUlcer. GastricDiscomfort DiarheaNOS. Flatulence. GastrointestinalDyskinesiaNOS. Indigestion.. EpigastricPain. EnlargedLingualPapilaNOS. LooseStool. AbdominalDiscomfort. AbdominalDistension Constipation. VomitingNOS (Continued)
12 . LiverDamageNOS HepatobiliaryDisorder 5.9 Pruritus Dermalor HypodermalDisorder. Erythema.. Eczema. SystemicPruritus. Rash. DrySkin. SwelingofSkin. MuscleRigidity Musculoskeletalor ConnectiveTissue Disorder. SwelingNOS. BackPain Hematuria RenalorUrinaryDisorder 5.9 AbnormalUrineNOS. UrinaryComplications. FrequentUrination.. Chils SystemicDisorderor SiteReactiondueto injection.. Dysesthesia.. Malaise. DryMouth LocalReactionNOS. LocalPain Fever.. SwelingNOS. PeripheralSweling. Asthenia 5.9 CRPIncrease ClinicalTests AST(GOT)Increase 9. ALT(GPT)Increase. IncreaseinAmmonia. DecreaseofHematocrit. DecreaseofHemoglobin 7.8 Increaseofγ -GTP(γ -GP).. IncreaseinBloodPressure. DecreaseinPlateletCount. IncreaseinSerum AI-P DecreaseinSerum Potassium.. DecreaseinSerum Cholesterol. DecreaseinSerum Sodium. IncreaseinSerum Bilirubin IncreaseinSerum LDH. IncreaseinEsinophilPercentage. DecreaseinEurythorcytes DecreaseinLeukocytes IncreaseinBodyWeight. CastinUrine GlucoseinUrine. BloodinUrine. ProteininUrine. DecreaseinUrineVolume. Self-InflictedWound Accident,Poisoning,or TreatmentComplication Table.(Continued)
13 Table. PlasmaconcentrationofITCZandoccurenceofadvancereactionatfinalevaluation FinalEvaluationPlasma ConcentrationofITCZ (Median:,79.ng/mL) GroupwithHighConcentrationofITCZ (abovemedian) GroupwithLowConcentrationofITCZ (belowmedian) Total Cases 5 9 Cases 7 7 Mild Grade ) Moderate Cases Cases Severe ) Whenonesubjectpresentedwithmorethanoneadvanceevent,themoreseverecasewasdocumented... Total Cases Table. Influenceofmedicationonrenalfunction CreatinineClearance(mL/min) TestPeriod RenalFunction Classification Subjects Priorto Administration After Administration Pairedt-test weekfolowing ITCZInjection Normal ModeratelyAbnormal 5.±8..9± ± 8. 8.±9. 7.±5. 7.±.5 p=. p=.8 p=. weeksfolowing ITCZInjection Normal ModeratelyAbnormal 9.9±.8 9.9± 7..±..±. 75.±.7 5.±.9 p=.7 p=. p=. CompletionofITCZ CapsuleAdministration Mean±S.D. BUN(mg/dL) TestPeriod RenalFunction Classification Subjects Priorto Administration After Administration Pairedt-test weekfolowing ITCZInjection Normal ModeratelyAbnormal.±.8 5.±.5 7.± ±..± 5. 7.± 5.5 p=. p=.77 p=.95 weeksfolowing ITCZInjection Normal ModeratelyAbnormal 5.±.8.7±. 7.±7..±..9±.9 5.7± 5.8 p=.9 p=.88 p=.7 CompletionofITCZ CapsuleAdministration Normal ModeratelyAbnormal 8.7±.8.±. 7.±7..5±.7.±..±.9 p=.9 p=.8 p=.89 Mean±S.D. Serum Creatinine(mg/dL) TestPeriod RenalFunction Classification Subjects Priorto Administration After Administration Pairedt-test weekfolowing ITCZInjection Normal ModeratelyAbnormal 7.7±..7±..±..7±..7±..9±. p=.9 p=.8 p=.9 weeksfolowing ITCZInjection Normal ModeratelyAbnormal 5 5.7±..7±..9±..±..±..9±. p=. p=. p=. weekfolowing ITCZ CapsuleAdministration Normal ModeratelyAbnormal 8.±..7±..9±..±..±..7±. p=. p=. p=. Mean±S.D.
14 Item Tested Table. Significantchangesinclinicalvaluesbeforeandafteradministration Preadministration- CompletionofA n Median( Min,Max) Hematological LeukocyteCount(/μ L) ITCZCapsuleAdministration - 95 (- 5,8,,8) p=. Test Rod-shapedCels ITCZCapsuleAdministration (, 9) p=.9 Biochemical BloodTest TotalCholesterol(mg/dL) Tryglyceride(mg/dL) TotalProtein(g/dL) UricAcid(mg/dL) Creatinine(mg/dL) TotalBilirubin(mg/dL) Sodium(mEq/L) Potassium(mEq/L) Chloride(mEq/L) Calcium(mEq/L) ITCZCapsuleAdministration ITCZCapsuleAdministration ITCZCapsuleAdministration ITCZCapsuleAdministration ITCZCapsuleAdministration ( - ( -. ( -.5 ( -. ( -. ( -.5 ( - 88, - 7, -., -.5, -., 7) ) ).8).) -.,.7) -.,.5). ( -.55, ( -. ( -. ( ( ( -, -.9, -.8,.) ).9) ) - 9,.) -,.7) -.5 ( -.75,.55) t-test p<. p=. p=.95 p=. p=. p=. p=. p=. p=. p=. p=. p=. p=. p=. Candida glabrata β
15
16 Aspergillus Candida Cryptococcus
日本化学療法学会雑誌第54巻第S-1号
 β β Key words β Candida krusei Candida glabrata β I β Drugadministration Baselinecharacteristics Clinicalsymptom Mycologicaltests Plasmadrugconcn Adverseevents Laboratorytests ) -leadecg Clinicalphotograph
β β Key words β Candida krusei Candida glabrata β I β Drugadministration Baselinecharacteristics Clinicalsymptom Mycologicaltests Plasmadrugconcn Adverseevents Laboratorytests ) -leadecg Clinicalphotograph
日本化学療法学会雑誌第56巻第3号
 β Key words Candida albicans albicans Candida C. albicans Candida glabrata Candida krusei albicans Candida C. glabrata C. albicans albicans Candida β in vitro in vivo C. glabrata C. krusei I β γ µ Candida
β Key words Candida albicans albicans Candida C. albicans Candida glabrata Candida krusei albicans Candida C. glabrata C. albicans albicans Candida β in vitro in vivo C. glabrata C. krusei I β γ µ Candida
日本化学療法学会雑誌第61巻第4号
 μ μ μ μ μ μ Key words I β μ Sex Age (years) Height (cm) Evaluation items Table1.Characteristics of patients 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) male 12 8 7 27 female 2 1
μ μ μ μ μ μ Key words I β μ Sex Age (years) Height (cm) Evaluation items Table1.Characteristics of patients 1. mg/kg/day (n14) 2.5 mg/kg/day (n9) 5. mg/kg/day (n9) Total (n32) male 12 8 7 27 female 2 1
日本化学療法学会雑誌第58巻第2号
 Key words β Candida Table1. MCFGCPAstudygroup Investigator (representative) YoshitsuguMiyazaki NaoyukiMiyashita RyoichiAmitani KenjiOgawa AtsuyukiKurashima ToshiroKiguchi MichiakiMishima YuichiInoue HiroshiSaito
Key words β Candida Table1. MCFGCPAstudygroup Investigator (representative) YoshitsuguMiyazaki NaoyukiMiyashita RyoichiAmitani KenjiOgawa AtsuyukiKurashima ToshiroKiguchi MichiakiMishima YuichiInoue HiroshiSaito
日本化学療法学会雑誌第59巻第5号
 Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S. pneumoniae H. influenzae M. catarrhalis S.
日本化学療法学会雑誌第54巻第S-1号
 β β β Key words β β I HP- -CD CHR RHC R R R R CHR R R R R RHC R R R CHR R R R RHC CHR R H CH CH Fig.. Structuralformulaofhydroxypropyl-β -cyclodextrin(hp-β -CD). CH n H β β β β Table-. Dosageandadministration(Singleadministrationstudy)
β β β Key words β β I HP- -CD CHR RHC R R R R CHR R R R R RHC R R R CHR R R R RHC CHR R H CH CH Fig.. Structuralformulaofhydroxypropyl-β -cyclodextrin(hp-β -CD). CH n H β β β β Table-. Dosageandadministration(Singleadministrationstudy)
日本化学療法学会雑誌第65巻第4号
 Mycoplasma pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae Key words Mycoplasma pneumoniae Mycoplasma pneumoniae M. pneumoniae I M. pneumoniae M. pneumoniae M. pneumoniae
Mycoplasma pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae M. pneumoniae Key words Mycoplasma pneumoniae Mycoplasma pneumoniae M. pneumoniae I M. pneumoniae M. pneumoniae M. pneumoniae
日本化学療法学会雑誌第57巻第S-2号
 Key words β I Table. Observationandtestschedule Testschedule Observation/Tests Beforetreatment Endoftreatment 5 9days aftercompletion oftreatment 4 6weeks aftercompletion oftreatment Informedconsent Patientbackground
Key words β I Table. Observationandtestschedule Testschedule Observation/Tests Beforetreatment Endoftreatment 5 9days aftercompletion oftreatment 4 6weeks aftercompletion oftreatment Informedconsent Patientbackground
日本化学療法学会雑誌第57巻第6号
 Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae S. pneumoniae β β H. influenzae S. pneumoniae H. influenzae M. catarrhalis Key words Otalgia Fever Item Crying,Iritation,
Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis S. pneumoniae S. pneumoniae β β H. influenzae S. pneumoniae H. influenzae M. catarrhalis Key words Otalgia Fever Item Crying,Iritation,
日本化学療法学会雑誌第55巻第S-1号
 µ µ Key words Streptococcus pneu- moniae S. pneumoniae µ µ I γ H H C HN F F O N O CO H H C SO H H O Fig.. ChemicalstructureofGRNX. γ γ II Haemophilus influenzae Pseudomonas aeruginosa Streptococcus intermedius
µ µ Key words Streptococcus pneu- moniae S. pneumoniae µ µ I γ H H C HN F F O N O CO H H C SO H H O Fig.. ChemicalstructureofGRNX. γ γ II Haemophilus influenzae Pseudomonas aeruginosa Streptococcus intermedius
日本化学療法学会雑誌第60巻第4号
 Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Key words β Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae μ μ S. pneumoniae H. influenzae S. pneumoniae
Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Key words β Streptococcus pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae μ μ S. pneumoniae H. influenzae S. pneumoniae
VOL.39 S-3
 VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
VOL.39 S-3 CHEMOTHERAPY SEPT.1991 Table 1. Background of characteristics and allocation of 5 healthy male volunteers in a multiple-dose study on panipenem/betamipron Day 1 Fig. 1. Schedule of multiple-dose
日本化学療法学会雑誌第56巻第1号
 β β β β β Streptococcus pneumoniaehaemophilus influenzaemoraxella catarrhalismycoplasma pneumoniaechlamydia pneumoniae β Key wordsβ mys nilc laci ngis Table. Assessmentschedule Parameters Patientcharacteristics
β β β β β Streptococcus pneumoniaehaemophilus influenzaemoraxella catarrhalismycoplasma pneumoniaechlamydia pneumoniae β Key wordsβ mys nilc laci ngis Table. Assessmentschedule Parameters Patientcharacteristics
日本化学療法学会雑誌第58巻第4号
 Escherichia coli Enterococcus faecalisstreptococcus agalactiae Klebsiella pneumoniae Staphylococcus epidermidis E. colie. faecalispseudomonas aeruginosa K. pneumoniae S. agalactiae E. coli E. coli μ p
Escherichia coli Enterococcus faecalisstreptococcus agalactiae Klebsiella pneumoniae Staphylococcus epidermidis E. colie. faecalispseudomonas aeruginosa K. pneumoniae S. agalactiae E. coli E. coli μ p
日本化学療法学会雑誌第57巻第5号
 in vitro Key words Streptococcus pneumoniae β Haemophilus influenzae Escherichia coli I Table. Antibacteriallevofloxacinactivityagainstclinicalisolates Organism Year Susceptibility(%)(Numberofstrains)
in vitro Key words Streptococcus pneumoniae β Haemophilus influenzae Escherichia coli I Table. Antibacteriallevofloxacinactivityagainstclinicalisolates Organism Year Susceptibility(%)(Numberofstrains)
CHEMOTHERAPY SEPT. 1991
 CHEMOTHERAPY SEPT. 1991 VOL. 39 S-3 Table 1. Background of characteristics and allocation of 9 healthy male volunteers in a single-dose study on betamipron Table 2. Background of characteristics and allocation
CHEMOTHERAPY SEPT. 1991 VOL. 39 S-3 Table 1. Background of characteristics and allocation of 9 healthy male volunteers in a single-dose study on betamipron Table 2. Background of characteristics and allocation
明海大学歯学雑誌 37‐2/1.秦泉寺
 J Meikai Dent Med 37, 153 158, 8 153 1 7 5ml /1 min Wong-Baker Face Rating Scale FS 5 1 9. g 3 5ml /1 min, FS 1 66 6ml /1 min FS 5 1 9. g 3 6ml /1 min, FS 3 Two Cases of Xerostomia that Showed an Improvement
J Meikai Dent Med 37, 153 158, 8 153 1 7 5ml /1 min Wong-Baker Face Rating Scale FS 5 1 9. g 3 5ml /1 min, FS 1 66 6ml /1 min FS 5 1 9. g 3 6ml /1 min, FS 3 Two Cases of Xerostomia that Showed an Improvement
日本化学療法学会雑誌第51巻第2号
 piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
piperacillin piperacillin PIPC. g Cmax CL PIPC CL CLR CLNR CL PIPC g g Cmax PIPC Key words: piperacillin Piperacillin PIPC PIPC g g PIPC Cmax g g ml g g ml g g ml T T T PIPC g g T Ccr ml min AUCCmax PIPC
日本化学療法学会雑誌第56巻第S-1号
 Key words Streptococcus pneumoniae µ µ H F Cl H H N N H N F COOH O Fig.1. Sitafloxacinstructure. I γ S. pneumoniae Haemophilus influenzae Table1. Observationandtestschedule Observation/Tests Informedconsent
Key words Streptococcus pneumoniae µ µ H F Cl H H N N H N F COOH O Fig.1. Sitafloxacinstructure. I γ S. pneumoniae Haemophilus influenzae Table1. Observationandtestschedule Observation/Tests Informedconsent
Key words : candidemia, endotoxin, D-arabinitol, Candida antigen, serological examination
 Key words : candidemia, endotoxin, D-arabinitol, Candida antigen, serological examination Fig. 1 The value of fungal index and D- arabinitol/creatinine. Fungal index was mesured by Endotoxin D and Endospecy
Key words : candidemia, endotoxin, D-arabinitol, Candida antigen, serological examination Fig. 1 The value of fungal index and D- arabinitol/creatinine. Fungal index was mesured by Endotoxin D and Endospecy
CHEMOTHERAPY APR. 1982
 VOL.30 S-1 CHEMOTHERAPY Table 1 Dose of CTT and subjects i. v.: Intravenous bolus injection d. i. v.: Intravenous drip infusion i. m.: Intramuscular injection Fig. 1 Schedule for examination of CTT, 1.0g
VOL.30 S-1 CHEMOTHERAPY Table 1 Dose of CTT and subjects i. v.: Intravenous bolus injection d. i. v.: Intravenous drip infusion i. m.: Intramuscular injection Fig. 1 Schedule for examination of CTT, 1.0g
1 見出し1,12ポ,日本語ゴシック,英語Arial,段落後は6ポの設定です
 8. Page1 8. B 1 5) 6) 2099 2370 50 400 mg 19 (1) 1) (a) 78.9% 30/38 4 3 35.7% 5/14 71.4% 10/1485.7% 12/142 1 83.3% 5/63 2 1 1 1 8. Page2 (b) 2099 1897 82.4% 1562/1897 2 84.0% 1298/1545 3 2 80.4% 472/58779.0%
8. Page1 8. B 1 5) 6) 2099 2370 50 400 mg 19 (1) 1) (a) 78.9% 30/38 4 3 35.7% 5/14 71.4% 10/1485.7% 12/142 1 83.3% 5/63 2 1 1 1 8. Page2 (b) 2099 1897 82.4% 1562/1897 2 84.0% 1298/1545 3 2 80.4% 472/58779.0%
400 46 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 Dec. 2012 LVFX 100 mg 3 / 7 150 mg 2 / 7 2 2006 2008 9 LVFX PK PD 2009 7 100 mg 1 3 500 mg 1 1 AUC/MIC
 Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
Dec. 2012 THE JAPANESE JOURNAL OF ANTIBIOTICS 65 6 399 45 2012 11 5 LVFX 500 mg 1 1 20 Chlamydia trachomatis C. trachomatismycoplasma genitalium M. genitalium LVFX 1 500 mg 1 1 7 22 22 C. trachomatis 17
Fig. 1 A: Effects of intramuscular injection of glucagon on the blood glucose levels (changes from basal, ƒ BG) as compared with effects of scopolamin
 Fig. 1 A: Effects of intramuscular injection of glucagon on the blood glucose levels (changes from basal, ƒ BG) as compared with effects of scopolamine butylbromide during upper gastrointestinal endoscopy.
Fig. 1 A: Effects of intramuscular injection of glucagon on the blood glucose levels (changes from basal, ƒ BG) as compared with effects of scopolamine butylbromide during upper gastrointestinal endoscopy.
Fig. 1 Chemical structure of norfioxacin (AM-715)
 Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
Fig. 1 Chemical structure of norfioxacin (AM-715) Table 1 Serum and biliary concentration of norfloxacin (AM-715) Table 2 Protocol for clinical evaluation of norfloxacin (AM-715) in the treatment of biliary
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, Sc
 A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
A Nutritional Study of Anemia in Pregnancy Hematologic Characteristics in Pregnancy (Part 1) Keizo Shiraki, Fumiko Hisaoka Department of Nutrition, School of Medicine, Tokushima University, Tokushima Fetal
 CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
CHEMOTHERAPY APRIL 1992 Table 2. Concentration of meropenem in human prostatic fluid Table 1. Background of 21 chronic complicated UTI cases * NB + BPH, NB + Kidney tumor, NB + Kidney tuberculosis Table
Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-
 Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl) oxy]-14-ethyl-12,
Fig. 1 Chemical structure of TE-031 Code number: TE-031 Chemical name: (-) (3R, 4S, 5S, 6R, 7R, 9R, 11R, 12R, 13S, 14R)-4-[(2, 6-dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl) oxy]-14-ethyl-12,
 CHEMOTHERAPY APRIL 1992 VOL. 40 S- 1 Table 1-1. Comparative in vitro activity of meropenem against clinical isolates CNS: coagulase-negative staphylococci CHEMOTHERAPY APRIL 1992 Table 1-2. Comparative
CHEMOTHERAPY APRIL 1992 VOL. 40 S- 1 Table 1-1. Comparative in vitro activity of meropenem against clinical isolates CNS: coagulase-negative staphylococci CHEMOTHERAPY APRIL 1992 Table 1-2. Comparative
 CHEMO THER APY Table 1. Background of the volunteers in the phase I clinical trial of ME1207 Table 2. Methods for Phase I clinical trial of ME1207 All doses are expressed in terms of potency as ME1206
CHEMO THER APY Table 1. Background of the volunteers in the phase I clinical trial of ME1207 Table 2. Methods for Phase I clinical trial of ME1207 All doses are expressed in terms of potency as ME1206
Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII (45)
 Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2305(45) 2306(46) THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 Dec. Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2307(47) 2308(48) THE JAPANESE
Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2305(45) 2306(46) THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 Dec. Dec. THE JAPANESE JOURNAL OF ANTIBIOTICS XXXVII-12 2307(47) 2308(48) THE JAPANESE
 Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
Table 1.Distribution and number of cases with acute upper respiratory tract infections classified according to antimicrobial agents administered Table 2. Distribution of cases which were enrolled to set
日本化学療法学会雑誌第57巻第4号
 Streptococcus pneumoniae Haemophilus influenzae β β Key words I β Enterococcus faecium Pseudomonas aeruginosa Streptococcus pneumoniae S. pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Table.
Streptococcus pneumoniae Haemophilus influenzae β β Key words I β Enterococcus faecium Pseudomonas aeruginosa Streptococcus pneumoniae S. pneumoniae Haemophilus influenzae S. pneumoniae H. influenzae Table.
051
 Aug. THE JAPANESE JOURNAL OF ANTIBIOTICS 58 4 389( 15 ) 1. MDS collagenase 2. 1 3. 4. / 390( 16 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 58 4 Aug. 4 4 2004 10 2 1 NTT Aug. THE JAPANESE JOURNAL OF ANTIBIOTICS
Aug. THE JAPANESE JOURNAL OF ANTIBIOTICS 58 4 389( 15 ) 1. MDS collagenase 2. 1 3. 4. / 390( 16 ) THE JAPANESE JOURNAL OF ANTIBIOTICS 58 4 Aug. 4 4 2004 10 2 1 NTT Aug. THE JAPANESE JOURNAL OF ANTIBIOTICS
1.8.2 Page MIC () MIC 50 / MIC 90 µg/ml Candida albicans (54) / Candida glabrata (25) 0.25 / 0.5 Candida guilliermondii a) (2)
 1.8.2 Page1 1.8.2 96% 2002 40 B 1 123 (1) in vitro 1) in vitro C. glabrata C. krusei 1 2 19951997 3 1999 2002 MIC MIC 2 1.8.2 Page2 1 19951997 MIC () MIC 50 / MIC 90 µg/ml Candida albicans (54) 0.016 /
1.8.2 Page1 1.8.2 96% 2002 40 B 1 123 (1) in vitro 1) in vitro C. glabrata C. krusei 1 2 19951997 3 1999 2002 MIC MIC 2 1.8.2 Page2 1 19951997 MIC () MIC 50 / MIC 90 µg/ml Candida albicans (54) 0.016 /
Table 1 Profiles of and number of clinical specimens obtain in febrile episodes *
 Key words: deep-seated mycosis, critical care center,(1 3)-ƒÀ-D-glucan, candida antigen Table 1 Profiles of and number of clinical specimens obtain in febrile episodes * Table 2 Underlying diseases of
Key words: deep-seated mycosis, critical care center,(1 3)-ƒÀ-D-glucan, candida antigen Table 1 Profiles of and number of clinical specimens obtain in febrile episodes * Table 2 Underlying diseases of
日本化学療法学会雑誌第57巻第S-2号
 µ µ Key words µ µ µ I Table1. Subjectprofilesin(A)singleand(B)multiple-dosestudies (A)Single-dosestudy Age (yr) Height (cm) Bodyweight (kg) BMI (kg/m ) Ccr(Cockcroft) (ml/min) Ethnicity dose(mg) n 5 5.1±.,3
µ µ Key words µ µ µ I Table1. Subjectprofilesin(A)singleand(B)multiple-dosestudies (A)Single-dosestudy Age (yr) Height (cm) Bodyweight (kg) BMI (kg/m ) Ccr(Cockcroft) (ml/min) Ethnicity dose(mg) n 5 5.1±.,3
感染症学雑誌第80巻第6号
 Candida Candida albicans Candida parapsilosis Candida glabrata Candida tropicalis C. albicans C. grabrata C. tropicalis µ Candida guilliermondii µc. parapsilosis µ Candida µcandida C. albicans µ µ µ µ
Candida Candida albicans Candida parapsilosis Candida glabrata Candida tropicalis C. albicans C. grabrata C. tropicalis µ Candida guilliermondii µc. parapsilosis µ Candida µcandida C. albicans µ µ µ µ
CHEMOTHERAPY Fig. 1 Chemical structure of CXM-AX
 Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
Fig. 1 Chemical structure of CXM-AX NOV. 1986 Fig. 2 Sensitivity distribution of clinical isolates organisms (106 cells/ml) a Smurcus 27 strains d) P.m irabilis 15 strains b Ecol i 27 strains 111.morganii
CHEMOTHERAPY JUN Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter cloacae 27, Proteus rettgeri 7, Proteus inconstans 20, Proteus
 VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
VOL. 32 S-4 CHEMOTHERAPY Fig. 1 Chemical structure of sodium cefoperazone Fig. 2 Chemical structure of sodium cefoperazone CHEMOTHERAPY JUN. 1984 Citrobacter freundii 27, Enterobacter aerogenes 26, Enterobacter
VOL.42 S-1
 CHEMOTHERAPY APR. 1994 VOL.42 S-1 CHEMOTHERAPY APR. 1994 Table 1. Criteria for evaluation of clinical efficacy by the Japanese Society of Oral and Maxillo-Facial Surgeons Grades of symptoms and numerical
CHEMOTHERAPY APR. 1994 VOL.42 S-1 CHEMOTHERAPY APR. 1994 Table 1. Criteria for evaluation of clinical efficacy by the Japanese Society of Oral and Maxillo-Facial Surgeons Grades of symptoms and numerical
Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution of female patients with blad
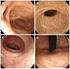 Key words: Female with bladder irritability, Subjective symptoms, Pyuria, Bacteriuria Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution
Key words: Female with bladder irritability, Subjective symptoms, Pyuria, Bacteriuria Fig. 1 Clinical findings and extent of inflammation area in female urethrocystitis Fig. 2 Classification and distribution
日本化学療法学会雑誌第54巻第1号
 Chlamydophila pneumoniae Chlamydophila psittaci Mycoplasma pneumoniae Legionella pneumophila C. pneumoniae C. psittaci C. pneumoniae C. psittaci M. pneumoniae M. pneumoniae C. psittaci L. pneumophila C.
Chlamydophila pneumoniae Chlamydophila psittaci Mycoplasma pneumoniae Legionella pneumophila C. pneumoniae C. psittaci C. pneumoniae C. psittaci M. pneumoniae M. pneumoniae C. psittaci L. pneumophila C.
Fig. 1 Chemical structure of Flurbiprofen Molecular formula : C1S H13 F02 Molecular weight : Chemical name : 2-( 2 -fluoro - 4 -biphenyly1 ) pr
 Key words : Flurbiprofen, Acute Upper Respiratory Tract Inflammation, Double-Blind Clinical Evaluation Fig. 1 Chemical structure of Flurbiprofen Molecular formula : C1S H13 F02 Molecular weight : 244.27
Key words : Flurbiprofen, Acute Upper Respiratory Tract Inflammation, Double-Blind Clinical Evaluation Fig. 1 Chemical structure of Flurbiprofen Molecular formula : C1S H13 F02 Molecular weight : 244.27
untitled
 1% 1 mg 1 mg 2 CTD 6 Section 2.6.1 Introduction 2.6.1 H 1 descarboethoxyloratadinesch 34117 7.9 SCH 34117 2.6.1-1 O OC 2 H 5 Cl N N JAN Loratadine (JANINN) ethyl 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]
1% 1 mg 1 mg 2 CTD 6 Section 2.6.1 Introduction 2.6.1 H 1 descarboethoxyloratadinesch 34117 7.9 SCH 34117 2.6.1-1 O OC 2 H 5 Cl N N JAN Loratadine (JANINN) ethyl 4-(8-chloro-5,6-dihydro-11H-benzo[5,6]
The Japanese Journal of Health Psychology, 29(S): (2017)
 Journal of Health Psychology Research 2017, Vol. 29, Special issue, 139 149Journal of Health Psychology Research 2016, J-STAGE Vol. Advance 29, Special publication issue, 139 149 date : 5 December, 2016
Journal of Health Psychology Research 2017, Vol. 29, Special issue, 139 149Journal of Health Psychology Research 2016, J-STAGE Vol. Advance 29, Special publication issue, 139 149 date : 5 December, 2016
 EVALUATION OF NOCTURNAL PENILE TUMESCENCE (NPT) IN THE DIFFERENTIAL DIAGNOSIS OF IMPOTENCE Masaharu Aoki, Yoshiaki Kumamoto, Kazutomi Mohri and Kazunori Ohno Department of Urology, Sapporo Medical College
EVALUATION OF NOCTURNAL PENILE TUMESCENCE (NPT) IN THE DIFFERENTIAL DIAGNOSIS OF IMPOTENCE Masaharu Aoki, Yoshiaki Kumamoto, Kazutomi Mohri and Kazunori Ohno Department of Urology, Sapporo Medical College
50mg 75mg (1) H 3 C O OH HO H O O H OH SO H 3 Na H H N H 2 N NH OH H N O O H H O OH H 3 C H O O N H H H HO H NH CH 3 HN HO H OH O H OH H NH
 3240 14 8 8 50mg 75mg 13 6 25 1-(1) H 3 C O OH HO H O O H OH SO H 3 Na H H N H 2 N NH OH H N O O H H O OH H 3 C H O O N H H H HO H NH CH 3 HN HO H OH O H OH H NH O N O C 56 H 70 N 9 NaO 23 S 1292.26 5-[(1S,2S)-2-
3240 14 8 8 50mg 75mg 13 6 25 1-(1) H 3 C O OH HO H O O H OH SO H 3 Na H H N H 2 N NH OH H N O O H H O OH H 3 C H O O N H H H HO H NH CH 3 HN HO H OH O H OH H NH O N O C 56 H 70 N 9 NaO 23 S 1292.26 5-[(1S,2S)-2-
Table 1 Eight clinical cases with candidemia gra.; granulocytopenia (<400/ml) before candidemia AMoL; acute monocytic leukemia VH; intravenous yperali
 Key words: candidemia, HA-test, Cand-Tec, D-arabinitol, Ĉ-d-glucan Table 1 Eight clinical cases with candidemia gra.; granulocytopenia (
Key words: candidemia, HA-test, Cand-Tec, D-arabinitol, Ĉ-d-glucan Table 1 Eight clinical cases with candidemia gra.; granulocytopenia (
VOL.27 CHEMOT S-1 HERAPY 185 に感 性 を示 して い たが,臨 床 的 に は無 効 で あ った 本 症 る 細 菌学 的 に も検 討 した が,起 炎菌 と考 え られ る細 菌 を 例 で は 基礎 疾 患 と して縦 隔 洞腫 瘍 が あ り,既 に 副 腎
 CHEMOTHERAPY Table 1 Clinical evaluation of Mezlocillin treatment against respiratory and other infectious diseases *: fever, eruption, granulocytopenia, thrombocytopenia, GOT, GPT elevation (See table
CHEMOTHERAPY Table 1 Clinical evaluation of Mezlocillin treatment against respiratory and other infectious diseases *: fever, eruption, granulocytopenia, thrombocytopenia, GOT, GPT elevation (See table
05_藤田先生_責
 This report shows innovation of competency of our faculty of social welfare. The aim of evaluation competency is improvement in the Social welfare education effects, by understanding of studentʼs development
This report shows innovation of competency of our faculty of social welfare. The aim of evaluation competency is improvement in the Social welfare education effects, by understanding of studentʼs development
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates
 Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
Table 1. Antibacterial activitiy of grepafloxacin and other antibiotics against clinical isolates Table 2-1. Summary of patients treated with grepafloxacin for respiratory infection 1) Out: outpatient,
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S.
 Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
Key words : 7432-S, Oral cephem, Urinary tract infection Fig. 1. Chemical structure of 7432-S. Table 1. Clinical summary of acute uncomplicated cystitis patients treated with 7432-S UTI : Criteria by the
 Effects of a Mixing Tea Drink Containing Depolymerized. Sodium Alginate on Serum Total Cholesterol and Assessment of its Safety ABSTRACT A double blind placebo-controlled study was conducted in 100 subjects
Effects of a Mixing Tea Drink Containing Depolymerized. Sodium Alginate on Serum Total Cholesterol and Assessment of its Safety ABSTRACT A double blind placebo-controlled study was conducted in 100 subjects
日本消化器外科学会雑誌第31巻第7号
 Key words: obstructive jaundice, biliary drainage, bile refeeding, hepatectomy Fig. 1 Animal models a: experiment I b: experiment 2 silicon tube Fig. 2 Experimantal design Sham Ope J - l r bile duct Zweeks
Key words: obstructive jaundice, biliary drainage, bile refeeding, hepatectomy Fig. 1 Animal models a: experiment I b: experiment 2 silicon tube Fig. 2 Experimantal design Sham Ope J - l r bile duct Zweeks
Fig.1 Case 1,E.H.Clinical Course of Campylobacter Meningitis Table 1 Case 1.E.H.
 Key words: Campylobacter fetus,meningitis Fig.1 Case 1,E.H.Clinical Course of Campylobacter Meningitis Table 1 Case 1.E.H. 273 昭 和61年3月20日 Table 2 Case 2.T.N. Fig.3 Case2,T.N.Clinical Campylobacter Fig.2
Key words: Campylobacter fetus,meningitis Fig.1 Case 1,E.H.Clinical Course of Campylobacter Meningitis Table 1 Case 1.E.H. 273 昭 和61年3月20日 Table 2 Case 2.T.N. Fig.3 Case2,T.N.Clinical Campylobacter Fig.2
Microsoft PowerPoint - LITA-noreflow_English.pptx
 A case of no-reflow phenomenon during PCI to LIMA graft Iwatsuki-Minami hospital, Saitama, Japan Koichi Sano, Masaki Tsukagoshi, Yasunari Ueno, Seiichi Fukuda, Takayoshi Sato, Yasuyuki Maruyama 77 y.o.
A case of no-reflow phenomenon during PCI to LIMA graft Iwatsuki-Minami hospital, Saitama, Japan Koichi Sano, Masaki Tsukagoshi, Yasunari Ueno, Seiichi Fukuda, Takayoshi Sato, Yasuyuki Maruyama 77 y.o.
CHEMOTHERAPY Fig. 1 Body weight changes of pregnant mice treated orally with AM- 715 Day of sestation
 CHEMOTHERAPY CHEMOTHERAPY Fig. 1 Body weight changes of pregnant mice treated orally with AM- 715 Day of sestation CHEMOTHERAPY Table 1 Preliminaly test of AM- 715 1): Mean } SD *: Significant difference
CHEMOTHERAPY CHEMOTHERAPY Fig. 1 Body weight changes of pregnant mice treated orally with AM- 715 Day of sestation CHEMOTHERAPY Table 1 Preliminaly test of AM- 715 1): Mean } SD *: Significant difference
O1-1 O1-2 O1-3 O1-4 O1-5 O1-6
 O1-1 O1-2 O1-3 O1-4 O1-5 O1-6 O1-7 O1-8 O1-9 O1-10 O1-11 O1-12 O1-13 O1-14 O1-15 O1-16 O1-17 O1-18 O1-19 O1-20 O1-21 O1-22 O1-23 O1-24 O1-25 O1-26 O1-27 O1-28 O1-29 O1-30 O1-31 O1-32 O1-33 O1-34 O1-35
O1-1 O1-2 O1-3 O1-4 O1-5 O1-6 O1-7 O1-8 O1-9 O1-10 O1-11 O1-12 O1-13 O1-14 O1-15 O1-16 O1-17 O1-18 O1-19 O1-20 O1-21 O1-22 O1-23 O1-24 O1-25 O1-26 O1-27 O1-28 O1-29 O1-30 O1-31 O1-32 O1-33 O1-34 O1-35
Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date URL
 Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date 1958-01 URL http://hdl.handle.net/2433/111559 Right Type Departmental Bulletin
Title 泌尿器科領域に於ける17-Ketosteroidの研究 17-Ketosteroidの臨床的研究 第 III 篇 : 尿 Author(s) 卜部, 敏入 Citation 泌尿器科紀要 (1958), 4(1): 3-31 Issue Date 1958-01 URL http://hdl.handle.net/2433/111559 Right Type Departmental Bulletin
sick contact1l
 Clinical Question 2017 年 5 月 15 日 分野 : 内分泌テーマ : 治療 19 14 15 18 sick contact1l 170cm/68.8kg, GCSE1V1M5, HR: 120/min, BP: 104/60mmHg, RR: 30/min, SpO 2 : 96%, BT: 38.0 WBC Hb PLT CRP Cr BUN 23400 15.0 35.5
Clinical Question 2017 年 5 月 15 日 分野 : 内分泌テーマ : 治療 19 14 15 18 sick contact1l 170cm/68.8kg, GCSE1V1M5, HR: 120/min, BP: 104/60mmHg, RR: 30/min, SpO 2 : 96%, BT: 38.0 WBC Hb PLT CRP Cr BUN 23400 15.0 35.5
125 2 P 1st washout 2 PB P mg/dL nd washout 2 P 5.5mg/dL< mg/dL <2.5mg/dL P P 2 D D 3 Ca 10
 . (1) 125 1 125 Renagel PB-94 P intact-pth P 1 b c a b 1 18 2 3 3 3 1 P 4 D 1 5 6 7 2 1HIV 2 3 4 5Hb 8.0g/dL ALT 48IU/L 6 7 PB-94 440mg 403mg 1-196 125 2 P 1st washout 2 PB-94 1 2 4 4 P 2.5 5.5mg/dL 1
. (1) 125 1 125 Renagel PB-94 P intact-pth P 1 b c a b 1 18 2 3 3 3 1 P 4 D 1 5 6 7 2 1HIV 2 3 4 5Hb 8.0g/dL ALT 48IU/L 6 7 PB-94 440mg 403mg 1-196 125 2 P 1st washout 2 PB-94 1 2 4 4 P 2.5 5.5mg/dL 1
日本化学療法学会雑誌第65巻第3号
 μ Key words Chlamydia trachomatis C. trachomatis I Table1.Observation items, categories and scores Observation item Category Score Body temperature () Lower abdominal pain Uterine corpus tenderness Condition
μ Key words Chlamydia trachomatis C. trachomatis I Table1.Observation items, categories and scores Observation item Category Score Body temperature () Lower abdominal pain Uterine corpus tenderness Condition
日本消化器外科学会雑誌第25巻第11号
 Key words: intestinal nerve plexus, hypoxic perfusion, immunohistochemistry, 5-100 protein Table I (A) Sroo staining reactivity of nerve tissue normal partly mildly declined all mildly declined partly
Key words: intestinal nerve plexus, hypoxic perfusion, immunohistochemistry, 5-100 protein Table I (A) Sroo staining reactivity of nerve tissue normal partly mildly declined all mildly declined partly
不安障害研究, 9(1), 17-32, 2017
 9(1), 17 32, 2017 Panic and Agoraphobia Scale 1 2 3 2 2 2 2 4 4 1 2 3 4 Panic and Agoraphobia Scale PAS DSM-IV-TR APA, 2000 3 4 PAS PAS PAS Patient Global Impression Improvement PAS 1980 Diagnostic and
9(1), 17 32, 2017 Panic and Agoraphobia Scale 1 2 3 2 2 2 2 4 4 1 2 3 4 Panic and Agoraphobia Scale PAS DSM-IV-TR APA, 2000 3 4 PAS PAS PAS Patient Global Impression Improvement PAS 1980 Diagnostic and
Web Stamps 96 KJ Stamps Web Vol 8, No 1, 2004
 The Journal of the Japan Academy of Nursing Administration and Policies Vol 8, No 1, pp 43 _ 57, 2004 The Literature Review of the Japanese Nurses Job Satisfaction Research Which the Stamps-Ozaki Scale
The Journal of the Japan Academy of Nursing Administration and Policies Vol 8, No 1, pp 43 _ 57, 2004 The Literature Review of the Japanese Nurses Job Satisfaction Research Which the Stamps-Ozaki Scale
8 The Bulletin of Meiji University of Integrative Medicine API II 61 ASO X 11 7 X-4 6 X m 5 X-2 4 X 3 9 X 11 7 API 0.84 ASO X 1 1 MR-angio
 7-14 2010 1 1 1 2 1 1 1 2 Fontaine II ASO61 3 API ASO ASO ASO API API KKKKKKKKKK ASO Fontaine II API Received April 14, 2009; Accepted July 16, 2009 I arteriosclerosis obliterans: ASO ASO 50 70 1,2 Fontaine
7-14 2010 1 1 1 2 1 1 1 2 Fontaine II ASO61 3 API ASO ASO ASO API API KKKKKKKKKK ASO Fontaine II API Received April 14, 2009; Accepted July 16, 2009 I arteriosclerosis obliterans: ASO ASO 50 70 1,2 Fontaine
 Fig. 1 Mean body weight changes of male rats administered cefaclor orally on fertility study Table. 1 Serum biochemical analysis in male rats administered cefaclor orally on fertility study The values
Fig. 1 Mean body weight changes of male rats administered cefaclor orally on fertility study Table. 1 Serum biochemical analysis in male rats administered cefaclor orally on fertility study The values
<95DB8C9288E397C389C88A E696E6462>
 2011 Vol.60 No.4 p.332 338 Usefulness of regional education program for dietary salt reduction: Self-monitoring of urinary salt excretion Kenichiro YASUTAKE[1] Kayoko SAWANO[1] Shoko YAMAGUCHI[1] Hiroko
2011 Vol.60 No.4 p.332 338 Usefulness of regional education program for dietary salt reduction: Self-monitoring of urinary salt excretion Kenichiro YASUTAKE[1] Kayoko SAWANO[1] Shoko YAMAGUCHI[1] Hiroko
CHEMOTHERAPY FEB Table 1. Activity of cefpirome and others against clinical isolates
 VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
VOL.39 S-1 CHEMOTHERAPY FEB. 1981 Table 1. Activity of cefpirome and others against clinical isolates VOL.39 S-1 CHEMOTHERAPY FEB. 1991 72 M, 55.5 kg 66 F, 53 kg Chronic bronchitis Bronchopneumonia Peak
JJRM5005/04.短報.責了.indd
 Jpn J Rehabil Med 2013 ; : 319.327 1 2 3 4 5 6 Survey of the Electronic Medical Recoding System Used in Kaifukuki Rehabilitation Wards Hidekazu SUGAWARA, 1 Tetsutaro YAHATA, 2 Hideto OKAZAKI, 3 Mitsuhiro
Jpn J Rehabil Med 2013 ; : 319.327 1 2 3 4 5 6 Survey of the Electronic Medical Recoding System Used in Kaifukuki Rehabilitation Wards Hidekazu SUGAWARA, 1 Tetsutaro YAHATA, 2 Hideto OKAZAKI, 3 Mitsuhiro
名称未設定-1
 Study on Clinical Factors Affecting the Fungal Culture Test - Relevance of Dry Mouth - Yoshiko YAMAMURA, Yukihiro MOMOTA, Hideyuki TAKANO, Koichi KANI, Katsumi MOTEGI, Fumihiro MATSUMOTO, Masayuki AZUMA
Study on Clinical Factors Affecting the Fungal Culture Test - Relevance of Dry Mouth - Yoshiko YAMAMURA, Yukihiro MOMOTA, Hideyuki TAKANO, Koichi KANI, Katsumi MOTEGI, Fumihiro MATSUMOTO, Masayuki AZUMA
Fig. 1 Chemical structure of KW-1070
 Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
Fig. 1 Chemical structure of KW-1070 Fig. 2 Sensitivity distribution of clinical isolates Fig. 4 Sensitivity distribution of clinical isolates Fig. 3 Sensitivity distribution of clinical isolates Fig.
CHEMOTHERAPY
 CHEMOTHERAPY VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin CHEMOTHERAPY AUG. 1993 VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin Table 1. Comparative in vitro activity of teicoplanin
CHEMOTHERAPY VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin CHEMOTHERAPY AUG. 1993 VOL.41 S-2 Laboratory and clinical evaluation of teicoplanin Table 1. Comparative in vitro activity of teicoplanin
32Vol. 37(1), Jan Gastroenterol Endosc 1995; 37: Hideyuki AKAI Observation and Evaluation of Esophageal Varices Before and After Sclerothe
 32Vol. 37(1), Jan. 1995 Gastroenterol Endosc 1995; 37: 32-8. Hideyuki AKAI Observation and Evaluation of Esophageal Varices Before and After Sclerotherapy by Infrared-Ray Electronic (TREE). Figure 1 The
32Vol. 37(1), Jan. 1995 Gastroenterol Endosc 1995; 37: 32-8. Hideyuki AKAI Observation and Evaluation of Esophageal Varices Before and After Sclerotherapy by Infrared-Ray Electronic (TREE). Figure 1 The
untitled
 11-19 2012 1 2 3 30 2 Key words acupuncture insulated needle cervical sympathetick trunk thermography blood flow of the nasal skin Received September 12, 2011; Accepted November 1, 2011 I 1 2 1954 3 564-0034
11-19 2012 1 2 3 30 2 Key words acupuncture insulated needle cervical sympathetick trunk thermography blood flow of the nasal skin Received September 12, 2011; Accepted November 1, 2011 I 1 2 1954 3 564-0034
Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone
 Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone (inoculum size= 106 CFU/ml) (Ĉ-lactamase producer : 2 strains) Fig. 1. Sensitivity distribution of
Table 1. Antibacterial spectrum SBT ABPC ABPC CPZ : sulbactamiampicillin : ampicillin : cefoperazone (inoculum size= 106 CFU/ml) (Ĉ-lactamase producer : 2 strains) Fig. 1. Sensitivity distribution of
05-d m-4.03
 Feb. THE JAPANESE JOURNAL OF ANTIBIOTICS 58 1 51(51) ABPA(M) 1 ABPM ITCZ 2 ABPA ITCZ 3 ABPA ABPM ABPM 52(52) THE JAPANESE JOURNAL OF ANTIBIOTICS 58 1 Feb. 9 2004 3 6 4 Feb. THE JAPANESE JOURNAL OF ANTIBIOTICS
Feb. THE JAPANESE JOURNAL OF ANTIBIOTICS 58 1 51(51) ABPA(M) 1 ABPM ITCZ 2 ABPA ITCZ 3 ABPA ABPM ABPM 52(52) THE JAPANESE JOURNAL OF ANTIBIOTICS 58 1 Feb. 9 2004 3 6 4 Feb. THE JAPANESE JOURNAL OF ANTIBIOTICS
小児感染免疫第25巻第2号
 Vol. 25No. 2145 1,2 1 1 1 1 2 2 2 TFLX 9 TFLX 450 mg16.6 mgkg TFLX 30 kg TFLX 2009 10 TFLX 1 1 2 3,4 TFLX 9 22 6 18 19 TFLX 450 mg150 mg 1 1 1 3 16.6 mgkg 20 20 0 21 3 135.0 cm0.1 S.D. 27.0 kg0.8 S.D.
Vol. 25No. 2145 1,2 1 1 1 1 2 2 2 TFLX 9 TFLX 450 mg16.6 mgkg TFLX 30 kg TFLX 2009 10 TFLX 1 1 2 3,4 TFLX 9 22 6 18 19 TFLX 450 mg150 mg 1 1 1 3 16.6 mgkg 20 20 0 21 3 135.0 cm0.1 S.D. 27.0 kg0.8 S.D.
Fig.1 Histograms of the sum of glucose concentrations for venous whole blood(vg)during 75 g OGTT in normal control subjects(a)and outpatients(b) Table
 Fig.1 Histograms of the sum of glucose concentrations for venous whole blood(vg)during 75 g OGTT in normal control subjects(a)and outpatients(b) Table1 Whole blood capillary-and venous-glucose levels in
Fig.1 Histograms of the sum of glucose concentrations for venous whole blood(vg)during 75 g OGTT in normal control subjects(a)and outpatients(b) Table1 Whole blood capillary-and venous-glucose levels in
VOL.35 S-2 CHEMOTHERAPY Table 1 Sex and age distribution Table 2 Applications of treatment with carumonam Table 3 Concentration of carumonam in human
 CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
CHEMOTHERAPY Fig. 1 Chemical structure of carumonam Disodium(+)-(Z)-CCE1-(2-amino-4-thiazoly1)-2-[[(2S, -(carbamoyloxymethyl)-4-oxo-1-sulfonato-3-azetidinyll -2-oxoethylidene] amino] oxy] acetate 3S)-2
indd
 18 61 1 2011 症例報告 2010 5 13 2010 12 20 A Case of Varicella with Severe Back Pain in a Patient with Systemic Lupus Erythematosus RIE SAITO, KIORI YANO, FUMIKO KATSUSHIMA, HARUYO IWADATE, HIROKO KOBAYASHI,
18 61 1 2011 症例報告 2010 5 13 2010 12 20 A Case of Varicella with Severe Back Pain in a Patient with Systemic Lupus Erythematosus RIE SAITO, KIORI YANO, FUMIKO KATSUSHIMA, HARUYO IWADATE, HIROKO KOBAYASHI,
VOL. 34 S-2 CHEMOTH8RAPY 913
 VOL. 34 S-2 CHEMOTH8RAPY 913 914 CHEMOTHERAPY APR. 1986 Fig. 1 Chemical structure of T-2588 and T-2525 T- 2588 pivaloyloxymethyl (+ )- (6 R, 7 R)-7-[(Z)-2- (2-amino- 4-thiazolyl)-2-methox yiminoacetamido]-3-[(
VOL. 34 S-2 CHEMOTH8RAPY 913 914 CHEMOTHERAPY APR. 1986 Fig. 1 Chemical structure of T-2588 and T-2525 T- 2588 pivaloyloxymethyl (+ )- (6 R, 7 R)-7-[(Z)-2- (2-amino- 4-thiazolyl)-2-methox yiminoacetamido]-3-[(
 Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
Table 1 Patients with various renal function * Ccr, Creatinine clearance ml/min per 1. 48 m2 ** C.V.D., Cerebral vascular disease ; C.R F., Chronic renal failure ; H.D., Hemoclialysis ; D., Dialyzer ;
Table 1 Labor data on admission symptoms headache ptosis of left eyelids double vision administration piperacillin sodium 1g/day 5g/day Fig. 1 clinica
 Table 1 Labor data on admission symptoms headache ptosis of left eyelids double vision administration piperacillin sodium 1g/day 5g/day Fig. 1 clinical course Fig. 2 The 23rd sick day. Both T1-weighted
Table 1 Labor data on admission symptoms headache ptosis of left eyelids double vision administration piperacillin sodium 1g/day 5g/day Fig. 1 clinical course Fig. 2 The 23rd sick day. Both T1-weighted
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study
 CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
CHEMOTHERAPY JUNE 1993 Table 1. Background of patients in pharmacokinetic study VOL. 41 S 1 Table 2. Levels (Đg/ml or Đg/g) of S-1006 in serum, bile, and tissue (gallbladder) after oral administration
Therapy for Asthenopia in Cases of Convergence Insufficiency Hiroko TAKASAKI, C.O.J., Nobuko INAGAMI, C.O.J., and Kayoko TAKENAWA, C.O.J.. Orthoptic c
 Therapy for Asthenopia in Cases of Convergence Insufficiency Hiroko TAKASAKI, C.O.J., Nobuko INAGAMI, C.O.J., and Kayoko TAKENAWA, C.O.J.. Orthoptic clinic, Department of Ophthalmology, (Director: Prof
Therapy for Asthenopia in Cases of Convergence Insufficiency Hiroko TAKASAKI, C.O.J., Nobuko INAGAMI, C.O.J., and Kayoko TAKENAWA, C.O.J.. Orthoptic clinic, Department of Ophthalmology, (Director: Prof
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST
 Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
Key words : Adverse reactions, Egg allergy, IgG antibody, Mills allergy, FAST 13) Danaeus, A., Johansson, S. G. O., Foucard, T. & Ohman, S.: Clinical and immunogical aspects of food allergy in childhood.
日本化学療法学会雑誌第57巻第1号
 In vitro Streptococcus pneumoniae Escherichia coli in vitro Streptococcus pneumoniae Escherichia coli µs. pneumoniae E. coli µ Key words in vitrostreptococcus pneumoniae Escherichia coli Escherichia coli
In vitro Streptococcus pneumoniae Escherichia coli in vitro Streptococcus pneumoniae Escherichia coli µs. pneumoniae E. coli µ Key words in vitrostreptococcus pneumoniae Escherichia coli Escherichia coli
日本職業・災害医学会会誌第54巻第6号
 Reprint request: A CASE OF PRESENTED VARIOUS FUNCTIONAL DISTURBANCES AFTER AN INJURY Taketoshi NOGAKI, Nobusuke HOUCHI, Tomoko SUGIUCHI, Naohiko WATANABE and Hiroyuki ZUSHO Department of Otolaryngology,
Reprint request: A CASE OF PRESENTED VARIOUS FUNCTIONAL DISTURBANCES AFTER AN INJURY Taketoshi NOGAKI, Nobusuke HOUCHI, Tomoko SUGIUCHI, Naohiko WATANABE and Hiroyuki ZUSHO Department of Otolaryngology,
Table 1 Components of corn dietary fibers
 Effects of Purified Corn Dietary Fiber on Blood Glucose, and Serum and Liver Lipids in Normal Rats and Mice Sachie Ikegami *1, Saeko Ohsawa *1, Shouko Machida *2 and Akiko Hada *2 The National Institute
Effects of Purified Corn Dietary Fiber on Blood Glucose, and Serum and Liver Lipids in Normal Rats and Mice Sachie Ikegami *1, Saeko Ohsawa *1, Shouko Machida *2 and Akiko Hada *2 The National Institute
 Effects of Diet Class on Overweight Middle-aged Women Analysis of Nutritional Intake Pattern YAMAGUCHI Shizue*, MATSUMOTO Tomoko**, MIMURA Kan-ichi***, ASAI Hitoshi****, OKUDA Toyoko***** * O saka Shin-ai
Effects of Diet Class on Overweight Middle-aged Women Analysis of Nutritional Intake Pattern YAMAGUCHI Shizue*, MATSUMOTO Tomoko**, MIMURA Kan-ichi***, ASAI Hitoshi****, OKUDA Toyoko***** * O saka Shin-ai
 9) Reichen J, Paumgartner G: Relationship be- tween bile flow and Na+, K+-Adenosinetriphos- phatase in liver plasma membranes enriched in bile canaliculi. J Clin Invest 60: 429-434, 1977 10) Schaffner
9) Reichen J, Paumgartner G: Relationship be- tween bile flow and Na+, K+-Adenosinetriphos- phatase in liver plasma membranes enriched in bile canaliculi. J Clin Invest 60: 429-434, 1977 10) Schaffner
 Table 1 Method of administration Inactive placebo. Table 2 Critoria for overall ef4fficy rating by committee L Severity and scores of main symptoms 1) Acute tonsillitis (Peritonsillids, Peritonsillar abscess)
Table 1 Method of administration Inactive placebo. Table 2 Critoria for overall ef4fficy rating by committee L Severity and scores of main symptoms 1) Acute tonsillitis (Peritonsillids, Peritonsillar abscess)






