untitled
|
|
|
- ありおき のたけ
- 5 years ago
- Views:
Transcription
1
2 Cotets 1. Itroductio-1 (. Itroductio- 3. Itroductio
3 Itroductio-1 Overview of physics of eutro-rich uclei
4 LBLN Isotope Project, S =0 S =0
5 RI 007 ZeroDegree RIPS RRC RILAC ZeroDegree RARF (old) frc IRC BigRIPS SRC
6 [MeV] 007 Rh (Z=45) Tc 98 Nb Y Y Rb Br (Z=35) As Ga Cu Co 64 Ni Sb 14 S I Ag Xe I Fe Ni PID F1 38U + Be(5mm) at 345 MeV/, F1 : +-mm, Brho : 76 Ni r-process path A/Z=.5 Kr Se Ge Cd Pd Ru Z (Z=30) 70 Ni Te S (Z=50) 13 S 117 Rh Mo Zr (Z=40) 104 Zr Sr 91 Br 8 Ge 76 Z TOF(F3-F7) [s]
7 Ca H He Be BC Li O N Ne Na Mg Al Si F S P Ca Ar Cl K A=4 A=5 4 O+ 3 O+ N=8 N= 5 O 4 O
8 4 O 5 F 4 Si 5 Si MeV/c ) 4 F 4 Ne 4 Na 4 Al MeV/c ) 5 Ne 5 Na 5 Al 4 Mg 5 Mg Z Z 4 O 4 F+e - +ν 4 Si 4 Al+e + +ν 4 Si+e - 4 Al+ν(
9 0.17 /fm 3 ( /cm 3 ) B( Z, N) c v 1/ 3 = { Zm p + Nm M ( Z, N)} V A, r = r0 A Weizsacker-Bethe /3 Z ( N Z) B( Z, N) = av A as A ac asym +δ 1/3 A A a = 16 MeV, a = 17 MeV(~ a ), a = 0.70 MeV, a = s δ = ±,0 0 v c sym B / A ~ 8 MeV 3MeV Quiz Quiz a sym
10 Fermi Gas Model --- Ω 3 Ω = dr dp / h = 3 (π ) Fermi Mometum 1/ 3 kf = 3π Ω 3π PF = h Fermi Eergy h k E = F F m 9 π = hc 3 8 r0 ( 50 MeV/c) Mea Kietic eergy ad mometum A Ω 1/ 3 4πk ~ 30 MeV 3 F P F T = E ~ 0 MeV, P = 5 0 = 930 MeV/c k F 1/ 3 = (300 / r N=Z MeV/c ~ 190 MeV/c V U 0 a 3 Ω = a Quiz: Quiz: Fermi Ω dk = (π ) 0 ) ~ 3 4 πk 3 3 F U 0 x
11 Z Be BC Li He H N Ne O F N N=8 11 Be 11 Li 19 C 1 N=0 β + β - (Neutro Halo) small S ρ ~ exp(-r/λ)/r 11 Li 9 Li S =300keV 11 Be 10 Be S =504keV -S Quiz s l=1, S
12 N=7 N=8 11 Be, 11 Li 1s 0p Why?? (Talmi Ua) I.Hamamoto 006) I. Talmi ad I. Ua, PRL4, 469 (1960). Myo, Kato, Ikeda et al., Private Comm.)
13 β decay of 11 Li N.Aoi et al., 11 Li 11 Be*+e - +ν 11 Be(g.s.)+γ 10 Be++(γ) ft 3 Gamow Teller h π l =, O = στ mc G Ψf O Ψi 11 Be 1/ + 0p 1/ domiat) log ft =5.67(4)) p Ψ [ ] (1s ) β (0 ) 11 ( Li) = Core α + p β =51(6)%
14 V(r) Major shell + r - Woods Saxo Potetial) 1 U = ω ( ) + ( r) m r + C l s Dl Major Shell Itruder Major Shell Quiz Major Shell hω 41A /3 MeV
15 Borromea 9 Li 11 Li α He di-eutro or cigar? α α α α 3 α + + α + + J.M. Bag, M. Zhukov et al., Phys.Rep. 64, 7 (1996). Borromea
16 E T z = -1 T z = 0 T z = +1 0 pp p p (=d) T=1, S=0,L=eve 1 S 0 SE T=0, S=1,L=eve 3 S D 1 TE (Spi Triplet) B=.MeV) (Spi Siglet) (Virtual State) V(r) Hard core ~ fm ( S+1 L J ) T+S+L=odd for NN system 1 3 r OPEP ~100MeV Quiz:
17 Hard core ~ fm OPEP V ( OPEP) Oe-pio Exchage Potetial 1 f = mπ c [ Vc ( r)( σ1 σ)( τ1 τ ) + VTN( r) S1( τ1 τ ) ] 3 hc ( σ1 σ )( τ1 τ ) = 3 TE(d) 3 3 exp( µ r) 3 ( r) = 1+ +, 1 = ( σ1 )( σ ) ( σ1 σ ) µ ( µ ) S r r r r µ r r SE(,p,pp) S=0 S 1 =0 V TN ( τ1 τ ) π 1 3 r exp( µ r) 1 m V c ( r) =, = = 1.4fm (fm) µ r µ hπ c f = 0.08 hc π For both SE(,p,pp) & TE(d)
18 Na isotope σ I E F Neutro radii ( Proto radii p Isotope shift T. Suzuki et PRL75,341 (1995). A. Ozawa,T. Suzuki, I. Taihata et al.npa693,3(001). E F = S p S
19 ρ p r core p p Ski r ρ core p r halo p p p fm Quiz:
20 6 He- 6 He 1 Be 6 He P( 6 He) 1 Be p 6 He P( 6 He) M.Freer et al., Phys.Rev.Lett. 8, 1383 (1999). A.Saito, S.Shimoura et al., More molecular States!
21
22 Y.Kaada-Eyo ad H.Horiuchi PRC5,647(1995). H.Takemoto,H.Horiuchi ad A.Oo PRC63,034615(001). B N=8 He Li 17 B He Li 19 B
23 N=0 T.Motobayashi et al., Phys. Lett. B (1995). 3 Mg 3 Mg* 3 Mg 49MeV/ucleo (β = 0.31c) γ γ High Z Target (Pb) NaI Si πl = E 3 Mg Doppler
24 3 Mg σ B c ( E) N( E) 4π β = B( E) 3ZeR R = R (1 + βy E ( 0 0 θ )) β = 0.51 ± B ( E) = 454 ± 78e N=0 fm 4 N=0 34 Mg H.Iwasaki et al.,plb5,7(001).
25 ,, ) ( 1 1 ) ( ) ( i f i m M M i i f f I El O I I M I Elm O M I El B f i + = = [ ] ) ( 1)!! ( 1) ( 8 1 El B c E l l l T l+ + + h h γ π (El) B reduced trasitio probability) f f i i M I M I l fm e =1/ = Ω = Z k l m l r k Y Ze Elm O 1 ) ( ) ( Wiger Eckart s Theorem ) ( ) ( 1 1 ) ( f f i i i f f i i f f M M lm I I I El O I I M I Elm O M I + = El Reduced Matrix elemet C.G. (
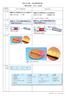
26 48 Ca beam 64MeV/ ~140pA RIPS Z Z Na (a) 34 Ne 31 F A/Z 43 Si (b) A/Z M.Notai, H.Sakurai et al. PLB54,49(00). Figure 1 Notai et al.
27 Implicatio of New isotopes Magicity Loss N~0 P Si Al Islad of Iversio 40 Al 41 Al 43 Si Mg Na 3 Mg 34 Mg Mg Mg 37 Na N=8 Ne 31 Ne 34 Ne F 31 F Z=8 O N 4 O 3 N Stability Jump Betwee O ad F Isotopes C C N=0 New Isotopes Uboud proved
28 16 C decouplig N.Imai et al. Phys.Rev.Lett. 9, (004) MeV 16 C + 0 +
29 16 O 16 C N.Imai et al. Phys.Rev.Lett. 9, (004). Y.Kaada-Eyo, PRC71,014303(005) H.J.Og et al., PRC73, 04610(006). 16 C(p,p ) eutro deformatio domiat
30 Summary 16 C H He Be BC Li N= O N Ne Na Mg Al Si F N=8 S P Ar Ca Cl K 1 Be N=8 N=16 N=0 Motobayashi,Sakurai et al. Ozawa et al. N=8
31
untitled
 --- = ---- 16 Z 8 0 8 8 0 Big Bang 8 8 s-process 50 r-process 8 50 N r-process s-process Hydrogen 71% Helium 8% Others 1.9% Heay 4-4% lements(>ni p-process (γ process? r-process s-process Big Bang H,He
--- = ---- 16 Z 8 0 8 8 0 Big Bang 8 8 s-process 50 r-process 8 50 N r-process s-process Hydrogen 71% Helium 8% Others 1.9% Heay 4-4% lements(>ni p-process (γ process? r-process s-process Big Bang H,He
03J_sources.key
 Radiation Detection & Measurement (1) (2) (3) (4)1 MeV ( ) 10 9 m 10 7 m 10 10 m < 10 18 m X 10 15 m 10 15 m ......... (isotope)...... (isotone)......... (isobar) 1 1 1 0 1 2 1 2 3 99.985% 0.015% ~0% E
Radiation Detection & Measurement (1) (2) (3) (4)1 MeV ( ) 10 9 m 10 7 m 10 10 m < 10 18 m X 10 15 m 10 15 m ......... (isotope)...... (isotone)......... (isobar) 1 1 1 0 1 2 1 2 3 99.985% 0.015% ~0% E
IS(A3) 核データ表 ( 内部転換 オージェ電子 ) No.e1 By IsoShieldJP 番号 核種核種半減期エネルギー放出割合核種番号通番数値単位 (kev) (%) 核崩壊型 娘核種 MG H β-/ce K A
 IS(A3)- 284 - No.e1 核種核種半減期エネルギー放出割合核種通番数値単位 (kev) (%) 1 1 1 MG-28 20.915 H 29.08 27.0000 β-/ce K Al-28 2 1 2 MG-28 20.915 H 30.64 2.6000 β-/ce L Al-28 3 2 1 SC-44M 58.6 H 270.84 0.0828 EC/CE CA-44 4 2
IS(A3)- 284 - No.e1 核種核種半減期エネルギー放出割合核種通番数値単位 (kev) (%) 1 1 1 MG-28 20.915 H 29.08 27.0000 β-/ce K Al-28 2 1 2 MG-28 20.915 H 30.64 2.6000 β-/ce L Al-28 3 2 1 SC-44M 58.6 H 270.84 0.0828 EC/CE CA-44 4 2
untitled
 N0 N8 N0 N8 N0 * 49MeV/nuceon β 0.3c γ Hgh Z Target Pb Equvaent Photon Method 0 d σ dωde σ π γ γ π E 3 γ [!! ] dnπ σ dω π γ Eγ c h C.A.Bertuan and G.Baur Phys.Rep.63,99 988. J.D. Jackson Cassca Eectrodynamcs
N0 N8 N0 N8 N0 * 49MeV/nuceon β 0.3c γ Hgh Z Target Pb Equvaent Photon Method 0 d σ dωde σ π γ γ π E 3 γ [!! ] dnπ σ dω π γ Eγ c h C.A.Bertuan and G.Baur Phys.Rep.63,99 988. J.D. Jackson Cassca Eectrodynamcs
42 3 u = (37) MeV/c 2 (3.4) [1] u amu m p m n [1] m H [2] m p = (4) MeV/c 2 = (13) u m n = (4) MeV/c 2 =
![42 3 u = (37) MeV/c 2 (3.4) [1] u amu m p m n [1] m H [2] m p = (4) MeV/c 2 = (13) u m n = (4) MeV/c 2 = 42 3 u = (37) MeV/c 2 (3.4) [1] u amu m p m n [1] m H [2] m p = (4) MeV/c 2 = (13) u m n = (4) MeV/c 2 =](/thumbs/73/68712188.jpg) 3 3.1 3.1.1 kg m s J = kg m 2 s 2 MeV MeV [1] 1MeV=1 6 ev = 1.62 176 462 (63) 1 13 J (3.1) [1] 1MeV/c 2 =1.782 661 731 (7) 1 3 kg (3.2) c =1 MeV (atomic mass unit) 12 C u = 1 12 M(12 C) (3.3) 41 42 3 u
3 3.1 3.1.1 kg m s J = kg m 2 s 2 MeV MeV [1] 1MeV=1 6 ev = 1.62 176 462 (63) 1 13 J (3.1) [1] 1MeV/c 2 =1.782 661 731 (7) 1 3 kg (3.2) c =1 MeV (atomic mass unit) 12 C u = 1 12 M(12 C) (3.3) 41 42 3 u
36 th IChO : - 3 ( ) , G O O D L U C K final 1
 36 th ICh - - 5 - - : - 3 ( ) - 169 - -, - - - - - - - G D L U C K final 1 1 1.01 2 e 4.00 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 16.00 9 F 19.00 10 Ne 20.18 11 Na 22.99 12 Mg 24.31 Periodic
36 th ICh - - 5 - - : - 3 ( ) - 169 - -, - - - - - - - G D L U C K final 1 1 1.01 2 e 4.00 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 16.00 9 F 19.00 10 Ne 20.18 11 Na 22.99 12 Mg 24.31 Periodic
(e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ,µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R,µ R,τ R (2.1a
![(e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ,µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R,µ R,τ R (2.1a (e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ,µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R,µ R,τ R (2.1a](/thumbs/100/144882653.jpg) 1 2 2.1 (e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ,µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R,µ R,τ R (2.1a) L ( ) ) * 2) W Z 1/2 ( - ) d u + e + ν e 1 1 0 0
1 2 2.1 (e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ,µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R,µ R,τ R (2.1a) L ( ) ) * 2) W Z 1/2 ( - ) d u + e + ν e 1 1 0 0
1 1 H Li Be Na M g B A l C S i N P O S F He N Cl A e K Ca S c T i V C Mn Fe Co Ni Cu Zn Ga Ge As Se B K Rb S Y Z Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb T e
 No. 1 1 1 H Li Be Na M g B A l C S i N P O S F He N Cl A e K Ca S c T i V C Mn Fe Co Ni Cu Zn Ga Ge As Se B K Rb S Y Z Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb T e I X e Cs Ba F Ra Hf Ta W Re Os I Rf Db Sg Bh
No. 1 1 1 H Li Be Na M g B A l C S i N P O S F He N Cl A e K Ca S c T i V C Mn Fe Co Ni Cu Zn Ga Ge As Se B K Rb S Y Z Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb T e I X e Cs Ba F Ra Hf Ta W Re Os I Rf Db Sg Bh
positron 1930 Dirac 1933 Anderson m 22Na(hl=2.6years), 58Co(hl=71days), 64Cu(hl=12hour) 68Ge(hl=288days) MeV : thermalization m psec 100
 positron 1930 Dirac 1933 Anderson m 22Na(hl=2.6years), 58Co(hl=71days), 64Cu(hl=12hour) 68Ge(hl=288days) 0.5 1.5MeV : thermalization 10 100 m psec 100psec nsec E total = 2mc 2 + E e + + E e Ee+ Ee-c mc
positron 1930 Dirac 1933 Anderson m 22Na(hl=2.6years), 58Co(hl=71days), 64Cu(hl=12hour) 68Ge(hl=288days) 0.5 1.5MeV : thermalization 10 100 m psec 100psec nsec E total = 2mc 2 + E e + + E e Ee+ Ee-c mc
6 2 T γ T B (6.4) (6.1) [( d nm + 3 ] 2 nt B )a 3 + nt B da 3 = 0 (6.9) na 3 = T B V 3/2 = T B V γ 1 = const. or T B a 2 = const. (6.10) H 2 = 8π kc2
![6 2 T γ T B (6.4) (6.1) [( d nm + 3 ] 2 nt B )a 3 + nt B da 3 = 0 (6.9) na 3 = T B V 3/2 = T B V γ 1 = const. or T B a 2 = const. (6.10) H 2 = 8π kc2 6 2 T γ T B (6.4) (6.1) [( d nm + 3 ] 2 nt B )a 3 + nt B da 3 = 0 (6.9) na 3 = T B V 3/2 = T B V γ 1 = const. or T B a 2 = const. (6.10) H 2 = 8π kc2](/thumbs/92/109118076.jpg) 1 6 6.1 (??) (P = ρ rad /3) ρ rad T 4 d(ρv ) + PdV = 0 (6.1) dρ rad ρ rad + 4 da a = 0 (6.2) dt T + da a = 0 T 1 a (6.3) ( ) n ρ m = n (m + 12 ) m v2 = n (m + 32 ) T, P = nt (6.4) (6.1) d [(nm + 32 ] )a
1 6 6.1 (??) (P = ρ rad /3) ρ rad T 4 d(ρv ) + PdV = 0 (6.1) dρ rad ρ rad + 4 da a = 0 (6.2) dt T + da a = 0 T 1 a (6.3) ( ) n ρ m = n (m + 12 ) m v2 = n (m + 32 ) T, P = nt (6.4) (6.1) d [(nm + 32 ] )a
( ) Note (e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ, µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) 3 * 2) [ ] [ ] [ ] ν e ν µ ν τ e
![( ) Note (e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ, µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) 3 * 2) [ ] [ ] [ ] ν e ν µ ν τ e ( ) Note (e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ, µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) 3 * 2) [ ] [ ] [ ] ν e ν µ ν τ e](/thumbs/98/136160482.jpg) ( ) Note 3 19 12 13 8 8.1 (e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ, µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) 3 * 2) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R, µ R, τ R (1a) L ( ) ) * 3) W Z 1/2 ( - )
( ) Note 3 19 12 13 8 8.1 (e ) (µ ) (τ ) ( (ν e,e ) e- (ν µ, µ ) µ- (ν τ,τ ) τ- ) ( ) ( ) (SU(2) ) (W +,Z 0,W ) * 1) 3 * 2) [ ] [ ] [ ] ν e ν µ ν τ e µ τ, e R, µ R, τ R (1a) L ( ) ) * 3) W Z 1/2 ( - )
23 1 Section ( ) ( ) ( 46 ) , 238( 235,238 U) 232( 232 Th) 40( 40 K, % ) (Rn) (Ra). 7( 7 Be) 14( 14 C) 22( 22 Na) (1 ) (2 ) 1 µ 2 4
 23 1 Section 1.1 1 ( ) ( ) ( 46 ) 2 3 235, 238( 235,238 U) 232( 232 Th) 40( 40 K, 0.0118% ) (Rn) (Ra). 7( 7 Be) 14( 14 C) 22( 22 Na) (1 ) (2 ) 1 µ 2 4 2 ( )2 4( 4 He) 12 3 16 12 56( 56 Fe) 4 56( 56 Ni)
23 1 Section 1.1 1 ( ) ( ) ( 46 ) 2 3 235, 238( 235,238 U) 232( 232 Th) 40( 40 K, 0.0118% ) (Rn) (Ra). 7( 7 Be) 14( 14 C) 22( 22 Na) (1 ) (2 ) 1 µ 2 4 2 ( )2 4( 4 He) 12 3 16 12 56( 56 Fe) 4 56( 56 Ni)
untitled
 71 7 3,000 1 MeV t = 1 MeV = c 1 MeV c 200 MeV fm 1 MeV 3.0 10 8 10 15 fm/s 0.67 10 21 s (1) 1fm t = 1fm c 1fm 3.0 10 8 10 15 fm/s 0.33 10 23 s (2) 10 22 s 7.1 ( ) a + b + B(+X +...) (3) a b B( X,...)
71 7 3,000 1 MeV t = 1 MeV = c 1 MeV c 200 MeV fm 1 MeV 3.0 10 8 10 15 fm/s 0.67 10 21 s (1) 1fm t = 1fm c 1fm 3.0 10 8 10 15 fm/s 0.33 10 23 s (2) 10 22 s 7.1 ( ) a + b + B(+X +...) (3) a b B( X,...)
(1.2) T D = 0 T = D = 30 kn 1.2 (1.4) 2F W = 0 F = W/2 = 300 kn/2 = 150 kn 1.3 (1.9) R = W 1 + W 2 = = 1100 N. (1.9) W 2 b W 1 a = 0
 1 1 1.1 1.) T D = T = D = kn 1. 1.4) F W = F = W/ = kn/ = 15 kn 1. 1.9) R = W 1 + W = 6 + 5 = 11 N. 1.9) W b W 1 a = a = W /W 1 )b = 5/6) = 5 cm 1.4 AB AC P 1, P x, y x, y y x 1.4.) P sin 6 + P 1 sin 45
1 1 1.1 1.) T D = T = D = kn 1. 1.4) F W = F = W/ = kn/ = 15 kn 1. 1.9) R = W 1 + W = 6 + 5 = 11 N. 1.9) W b W 1 a = a = W /W 1 )b = 5/6) = 5 cm 1.4 AB AC P 1, P x, y x, y y x 1.4.) P sin 6 + P 1 sin 45
1/120 別表第 1(6 8 及び10 関係 ) 放射性物質の種類が明らかで かつ 一種類である場合の放射線業務従事者の呼吸する空気中の放射性物質の濃度限度等 添付 第一欄第二欄第三欄第四欄第五欄第六欄 放射性物質の種類 吸入摂取した 経口摂取した 放射線業 周辺監視 周辺監視 場合の実効線 場合
 1/120 別表第 1(6 8 及び10 関係 ) 放射性物質の種類が明らかで かつ 一種類である場合の放射線業務従事者の呼吸する空気中の放射性物質の濃度限度等 添付 第一欄第二欄第三欄第四欄第五欄第六欄 放射性物質の種類 吸入摂取した 経口摂取した 放射線業 周辺監視 周辺監視 場合の実効線 場合の実効線 務従事者 区域外の 区域外の 量係数 量係数 の呼吸す 空気中の 水中の濃 る空気中 濃度限度
1/120 別表第 1(6 8 及び10 関係 ) 放射性物質の種類が明らかで かつ 一種類である場合の放射線業務従事者の呼吸する空気中の放射性物質の濃度限度等 添付 第一欄第二欄第三欄第四欄第五欄第六欄 放射性物質の種類 吸入摂取した 経口摂取した 放射線業 周辺監視 周辺監視 場合の実効線 場合の実効線 務従事者 区域外の 区域外の 量係数 量係数 の呼吸す 空気中の 水中の濃 る空気中 濃度限度
H1-H4
 42 S H He Li H He Li Be B C N O F Ne Be B C N O F Ne H He Li Be B H H e L i Na Mg Al Si S Cl Ar Na Mg Al Si S Cl Ar C N O F Ne Na Be B C N O F Ne Na K Sc T i V C r K Sc Ti V Cr M n F e C o N i Mn Fe Mg
42 S H He Li H He Li Be B C N O F Ne Be B C N O F Ne H He Li Be B H H e L i Na Mg Al Si S Cl Ar Na Mg Al Si S Cl Ar C N O F Ne Na Be B C N O F Ne Na K Sc T i V C r K Sc Ti V Cr M n F e C o N i Mn Fe Mg
C el = 3 2 Nk B (2.14) c el = 3k B C el = 3 2 Nk B
 I ino@hiroshima-u.ac.jp 217 11 14 4 4.1 2 2.4 C el = 3 2 Nk B (2.14) c el = 3k B 2 3 3.15 C el = 3 2 Nk B 3.15 39 2 1925 (Wolfgang Pauli) (Pauli exclusion principle) T E = p2 2m p T N 4 Pauli Sommerfeld
I ino@hiroshima-u.ac.jp 217 11 14 4 4.1 2 2.4 C el = 3 2 Nk B (2.14) c el = 3k B 2 3 3.15 C el = 3 2 Nk B 3.15 39 2 1925 (Wolfgang Pauli) (Pauli exclusion principle) T E = p2 2m p T N 4 Pauli Sommerfeld
総研大恒星進化概要.dvi
 The Structure and Evolution of Stars I. Basic Equations. M r r =4πr2 ρ () P r = GM rρ. r 2 (2) r: M r : P and ρ: G: M r Lagrange r = M r 4πr 2 rho ( ) P = GM r M r 4πr. 4 (2 ) s(ρ, P ) s(ρ, P ) r L r T
The Structure and Evolution of Stars I. Basic Equations. M r r =4πr2 ρ () P r = GM rρ. r 2 (2) r: M r : P and ρ: G: M r Lagrange r = M r 4πr 2 rho ( ) P = GM r M r 4πr. 4 (2 ) s(ρ, P ) s(ρ, P ) r L r T
Chadwick [ 1 ] 1919,, electron number Q kinetic energy [MeV] 8.1: 8.1, 1 internal conversion electron E γ E e =
![Chadwick [ 1 ] 1919,, electron number Q kinetic energy [MeV] 8.1: 8.1, 1 internal conversion electron E γ E e = Chadwick [ 1 ] 1919,, electron number Q kinetic energy [MeV] 8.1: 8.1, 1 internal conversion electron E γ E e =](/thumbs/104/162189465.jpg) 8 8.1 8.1.1 1 Chadwick [ 1 ] 1919,, electron number Q 0.0 0. 0.4 0.6 0.8 1.0 kinetic energy [MeV] 8.1: 8.1, 1 internal conversion electron E γ E e = E γ φ φ E e X 153 154 8, 3 H 3 He, ( ) 3 H( 1 ) 3 He(
8 8.1 8.1.1 1 Chadwick [ 1 ] 1919,, electron number Q 0.0 0. 0.4 0.6 0.8 1.0 kinetic energy [MeV] 8.1: 8.1, 1 internal conversion electron E γ E e = E γ φ φ E e X 153 154 8, 3 H 3 He, ( ) 3 H( 1 ) 3 He(
RN201602_cs5_0122.indd
 ISSN 1349-1229 No.416 February 2016 2 SPECIAL TOPIC113 SPECIAL TOPIC 113 FACE Mykinso 113 SPECIAL TOPIC IUPAC 11320151231 RI RIBFRILAC 20039Zn30 Bi83 20047113 20054201283 113 1133 Bh107 20082009 113 113
ISSN 1349-1229 No.416 February 2016 2 SPECIAL TOPIC113 SPECIAL TOPIC 113 FACE Mykinso 113 SPECIAL TOPIC IUPAC 11320151231 RI RIBFRILAC 20039Zn30 Bi83 20047113 20054201283 113 1133 Bh107 20082009 113 113
物理化学I-第12回(13).ppt
 I- 12-1 11 11.1 2Mg(s) + O 2 (g) 2MgO(s) [Mg 2+ O 2 ] Zn(s) + Cu 2+ (aq) Zn 2+ (aq) + Cu(s) - 2Mg(s) 2Mg 2+ (s) + 4e +) O 2 (g) + 4e 2O 2 (s) 2Mg(s) + O 2 (g) 2MgO(s) Zn(s) Zn 2+ (aq) + 2e +) Cu 2+ (aq)
I- 12-1 11 11.1 2Mg(s) + O 2 (g) 2MgO(s) [Mg 2+ O 2 ] Zn(s) + Cu 2+ (aq) Zn 2+ (aq) + Cu(s) - 2Mg(s) 2Mg 2+ (s) + 4e +) O 2 (g) + 4e 2O 2 (s) 2Mg(s) + O 2 (g) 2MgO(s) Zn(s) Zn 2+ (aq) + 2e +) Cu 2+ (aq)
The Physics of Atmospheres CAPTER :
 The Physics of Atmospheres CAPTER 4 1 4 2 41 : 2 42 14 43 17 44 25 45 27 46 3 47 31 48 32 49 34 41 35 411 36 maintex 23/11/28 The Physics of Atmospheres CAPTER 4 2 4 41 : 2 1 σ 2 (21) (22) k I = I exp(
The Physics of Atmospheres CAPTER 4 1 4 2 41 : 2 42 14 43 17 44 25 45 27 46 3 47 31 48 32 49 34 41 35 411 36 maintex 23/11/28 The Physics of Atmospheres CAPTER 4 2 4 41 : 2 1 σ 2 (21) (22) k I = I exp(
4. ϵ(ν, T ) = c 4 u(ν, T ) ϵ(ν, T ) T ν π4 Planck dx = 0 e x 1 15 U(T ) x 3 U(T ) = σt 4 Stefan-Boltzmann σ 2π5 k 4 15c 2 h 3 = W m 2 K 4 5.
 A 1. Boltzmann Planck u(ν, T )dν = 8πh ν 3 c 3 kt 1 dν h 6.63 10 34 J s Planck k 1.38 10 23 J K 1 Boltzmann u(ν, T ) T ν e hν c = 3 10 8 m s 1 2. Planck λ = c/ν Rayleigh-Jeans u(ν, T )dν = 8πν2 kt dν c
A 1. Boltzmann Planck u(ν, T )dν = 8πh ν 3 c 3 kt 1 dν h 6.63 10 34 J s Planck k 1.38 10 23 J K 1 Boltzmann u(ν, T ) T ν e hν c = 3 10 8 m s 1 2. Planck λ = c/ν Rayleigh-Jeans u(ν, T )dν = 8πν2 kt dν c
元素分析
 : このマークが付してある著作物は 第三者が有する著作物ですので 同著作物の再使用 同著作物の二次的著作物の創作等については 著作権者より直接使用許諾を得る必要があります (PET) 1 18 1 18 H 2 13 14 15 16 17 He 1 2 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Na Mg 3 4 5 6 7 8 9 10 11 12 Al Si P
: このマークが付してある著作物は 第三者が有する著作物ですので 同著作物の再使用 同著作物の二次的著作物の創作等については 著作権者より直接使用許諾を得る必要があります (PET) 1 18 1 18 H 2 13 14 15 16 17 He 1 2 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Na Mg 3 4 5 6 7 8 9 10 11 12 Al Si P
反D中間子と核子のエキゾチックな 束縛状態と散乱状態の解析
 .... D 1 in collaboration with 1, 2, 1 RCNP 1, KEK 2 . Exotic hadron qqq q q Θ + Λ(1405) etc. uudd s? KN quasi-bound state? . D(B)-N bound state { { D D0 ( cu) B = D ( cd), B = + ( bu) B 0 ( bd) D(B)-N
.... D 1 in collaboration with 1, 2, 1 RCNP 1, KEK 2 . Exotic hadron qqq q q Θ + Λ(1405) etc. uudd s? KN quasi-bound state? . D(B)-N bound state { { D D0 ( cu) B = D ( cd), B = + ( bu) B 0 ( bd) D(B)-N
) ] [ h m x + y + + V x) φ = Eφ 1) z E = i h t 13) x << 1) N n n= = N N + 1) 14) N n n= = N N + 1)N + 1) 6 15) N n 3 n= = 1 4 N N + 1) 16) N n 4
![) ] [ h m x + y + + V x) φ = Eφ 1) z E = i h t 13) x << 1) N n n= = N N + 1) 14) N n n= = N N + 1)N + 1) 6 15) N n 3 n= = 1 4 N N + 1) 16) N n 4 ) ] [ h m x + y + + V x) φ = Eφ 1) z E = i h t 13) x << 1) N n n= = N N + 1) 14) N n n= = N N + 1)N + 1) 6 15) N n 3 n= = 1 4 N N + 1) 16) N n 4](/thumbs/92/107866707.jpg) 1. k λ ν ω T v p v g k = π λ ω = πν = π T v p = λν = ω k v g = dω dk 1) ) 3) 4). p = hk = h λ 5) E = hν = hω 6) h = h π 7) h =6.6618 1 34 J sec) hc=197.3 MeV fm = 197.3 kev pm= 197.3 ev nm = 1.97 1 3 ev
1. k λ ν ω T v p v g k = π λ ω = πν = π T v p = λν = ω k v g = dω dk 1) ) 3) 4). p = hk = h λ 5) E = hν = hω 6) h = h π 7) h =6.6618 1 34 J sec) hc=197.3 MeV fm = 197.3 kev pm= 197.3 ev nm = 1.97 1 3 ev
untitled
 NPO 2006( ) 11 14 ( ) (2006/12/3) 1 50% % - - (CO+H2) ( ) 6 44 1) --- 2) ( CO H2 ) 2 3 3 90 3 3 2 3 2004 ( ) 1 1 4 1 20% 5 ( ) ( ) 2 6 MAWERA ) MAWERA ( ) ( ) 7 6MW -- 175kW 8 ( ) 900 10 2 2 2 9 -- - 10
NPO 2006( ) 11 14 ( ) (2006/12/3) 1 50% % - - (CO+H2) ( ) 6 44 1) --- 2) ( CO H2 ) 2 3 3 90 3 3 2 3 2004 ( ) 1 1 4 1 20% 5 ( ) ( ) 2 6 MAWERA ) MAWERA ( ) ( ) 7 6MW -- 175kW 8 ( ) 900 10 2 2 2 9 -- - 10
( ) ,
 II 2007 4 0. 0 1 0 2 ( ) 0 3 1 2 3 4, - 5 6 7 1 1 1 1 1) 2) 3) 4) ( ) () H 2.79 10 10 He 2.72 10 9 C 1.01 10 7 N 3.13 10 6 O 2.38 10 7 Ne 3.44 10 6 Mg 1.076 10 6 Si 1 10 6 S 5.15 10 5 Ar 1.01 10 5 Fe 9.00
II 2007 4 0. 0 1 0 2 ( ) 0 3 1 2 3 4, - 5 6 7 1 1 1 1 1) 2) 3) 4) ( ) () H 2.79 10 10 He 2.72 10 9 C 1.01 10 7 N 3.13 10 6 O 2.38 10 7 Ne 3.44 10 6 Mg 1.076 10 6 Si 1 10 6 S 5.15 10 5 Ar 1.01 10 5 Fe 9.00
V(x) m e V 0 cos x π x π V(x) = x < π, x > π V 0 (i) x = 0 (V(x) V 0 (1 x 2 /2)) n n d 2 f dξ 2ξ d f 2 dξ + 2n f = 0 H n (ξ) (ii) H
 199 1 1 199 1 1. Vx) m e V cos x π x π Vx) = x < π, x > π V i) x = Vx) V 1 x /)) n n d f dξ ξ d f dξ + n f = H n ξ) ii) H n ξ) = 1) n expξ ) dn dξ n exp ξ )) H n ξ)h m ξ) exp ξ )dξ = π n n!δ n,m x = Vx)
199 1 1 199 1 1. Vx) m e V cos x π x π Vx) = x < π, x > π V i) x = Vx) V 1 x /)) n n d f dξ ξ d f dξ + n f = H n ξ) ii) H n ξ) = 1) n expξ ) dn dξ n exp ξ )) H n ξ)h m ξ) exp ξ )dξ = π n n!δ n,m x = Vx)
main.dvi
 SGC - 48 208X Y Z Z 2006 1930 β Z 2006! 1 2 3 Z 1930 SGC -12, 2001 5 6 http://www.saiensu.co.jp/support.htm http://www.shinshu-u.ac.jp/ haru/ xy.z :-P 3 4 2006 3 ii 1 1 1.1... 1 1.2 1930... 1 1.3 1930...
SGC - 48 208X Y Z Z 2006 1930 β Z 2006! 1 2 3 Z 1930 SGC -12, 2001 5 6 http://www.saiensu.co.jp/support.htm http://www.shinshu-u.ac.jp/ haru/ xy.z :-P 3 4 2006 3 ii 1 1 1.1... 1 1.2 1930... 1 1.3 1930...
4 2 Rutherford 89 Rydberg λ = R ( n 2 ) n 2 n = n +,n +2, n = Lyman n =2 Balmer n =3 Paschen R Rydberg R = cm 896 Zeeman Zeeman Zeeman Lorentz
 2 Rutherford 2. Rutherford N. Bohr Rutherford 859 Kirchhoff Bunsen 86 Maxwell Maxwell 885 Balmer λ Balmer λ = 364.56 n 2 n 2 4 Lyman, Paschen 3 nm, n =3, 4, 5, 4 2 Rutherford 89 Rydberg λ = R ( n 2 ) n
2 Rutherford 2. Rutherford N. Bohr Rutherford 859 Kirchhoff Bunsen 86 Maxwell Maxwell 885 Balmer λ Balmer λ = 364.56 n 2 n 2 4 Lyman, Paschen 3 nm, n =3, 4, 5, 4 2 Rutherford 89 Rydberg λ = R ( n 2 ) n
PowerPoint プレゼンテーション
 2004 3 3 2 3 4 5 6 7 8 9 10 T. Ito, A. Yamamoto, et al., J. Chem. Soc., Chem. Commun., 136 (1974) J. Chem. Soc., Dalton Trans., 1783 (1974) J. Chem. Soc., Dalton Trans., 1398 (1975) 11 T.Ito, A. Yamamoto,
2004 3 3 2 3 4 5 6 7 8 9 10 T. Ito, A. Yamamoto, et al., J. Chem. Soc., Chem. Commun., 136 (1974) J. Chem. Soc., Dalton Trans., 1783 (1974) J. Chem. Soc., Dalton Trans., 1398 (1975) 11 T.Ito, A. Yamamoto,
rcnp01may-2
 E22 RCP Ring-Cyclotron 97 953 K beam K-atom HF X K, +,K + e,e K + -spectroscopy OK U U I= First-order -exchange - coupling I= U LS U LS Meson-exchange model /5/ I= Symmetric LS Anti-symmetric LS ( σ Λ
E22 RCP Ring-Cyclotron 97 953 K beam K-atom HF X K, +,K + e,e K + -spectroscopy OK U U I= First-order -exchange - coupling I= U LS U LS Meson-exchange model /5/ I= Symmetric LS Anti-symmetric LS ( σ Λ
 http://radphys4.c.u-tokyo.ac.jp/~torii/lecture/radiolect-kn.html 21 KOMCEE K303 2013 / 10 / 18 / 21 KOMCEE K303 Billet de 500 Francs Français en circulation: 1993 1999 α β γ X VIDEO http://eneco.jaero.or.jp/20110322/
http://radphys4.c.u-tokyo.ac.jp/~torii/lecture/radiolect-kn.html 21 KOMCEE K303 2013 / 10 / 18 / 21 KOMCEE K303 Billet de 500 Francs Français en circulation: 1993 1999 α β γ X VIDEO http://eneco.jaero.or.jp/20110322/
LLG-R8.Nisus.pdf
 d M d t = γ M H + α M d M d t M γ [ 1/ ( Oe sec) ] α γ γ = gµ B h g g µ B h / π γ g = γ = 1.76 10 [ 7 1/ ( Oe sec) ] α α = λ γ λ λ λ α γ α α H α = γ H ω ω H α α H K K H K / M 1 1 > 0 α 1 M > 0 γ α γ =
d M d t = γ M H + α M d M d t M γ [ 1/ ( Oe sec) ] α γ γ = gµ B h g g µ B h / π γ g = γ = 1.76 10 [ 7 1/ ( Oe sec) ] α α = λ γ λ λ λ α γ α α H α = γ H ω ω H α α H K K H K / M 1 1 > 0 α 1 M > 0 γ α γ =
Canvas-tr01(title).cv3
 Working Group DaiMaJin DaiRittaikaku Multiparticle Jiki-Bunnsekiki Samurai7 Superconducting Analyser for Multi particles from RadioIsotope Beams with 7Tm of bending power (γ,n) softgdr, GDR non resonant
Working Group DaiMaJin DaiRittaikaku Multiparticle Jiki-Bunnsekiki Samurai7 Superconducting Analyser for Multi particles from RadioIsotope Beams with 7Tm of bending power (γ,n) softgdr, GDR non resonant
 006 11 8 0 3 1 5 1.1..................... 5 1......................... 6 1.3.................... 6 1.4.................. 8 1.5................... 8 1.6................... 10 1.6.1......................
006 11 8 0 3 1 5 1.1..................... 5 1......................... 6 1.3.................... 6 1.4.................. 8 1.5................... 8 1.6................... 10 1.6.1......................
Microsoft Word - 11問題表紙(選択).docx
 A B A.70g/cm 3 B.74g/cm 3 B C 70at% %A C B at% 80at% %B 350 C γ δ y=00 x-y ρ l S ρ C p k C p ρ C p T ρ l t l S S ξ S t = ( k T ) ξ ( ) S = ( k T) ( ) t y ξ S ξ / t S v T T / t = v T / y 00 x v S dy dx
A B A.70g/cm 3 B.74g/cm 3 B C 70at% %A C B at% 80at% %B 350 C γ δ y=00 x-y ρ l S ρ C p k C p ρ C p T ρ l t l S S ξ S t = ( k T ) ξ ( ) S = ( k T) ( ) t y ξ S ξ / t S v T T / t = v T / y 00 x v S dy dx
1 9 v.0.1 c (2016/10/07) Minoru Suzuki T µ 1 (7.108) f(e ) = 1 e β(e µ) 1 E 1 f(e ) (Bose-Einstein distribution function) *1 (8.1) (9.1)
 1 9 v..1 c (216/1/7) Minoru Suzuki 1 1 9.1 9.1.1 T µ 1 (7.18) f(e ) = 1 e β(e µ) 1 E 1 f(e ) (Bose-Einstein distribution function) *1 (8.1) (9.1) E E µ = E f(e ) E µ (9.1) µ (9.2) µ 1 e β(e µ) 1 f(e )
1 9 v..1 c (216/1/7) Minoru Suzuki 1 1 9.1 9.1.1 T µ 1 (7.18) f(e ) = 1 e β(e µ) 1 E 1 f(e ) (Bose-Einstein distribution function) *1 (8.1) (9.1) E E µ = E f(e ) E µ (9.1) µ (9.2) µ 1 e β(e µ) 1 f(e )
Mott散乱によるParity対称性の破れを検証
 Mott Parity P2 Mott target Mott Parity Parity Γ = 1 0 0 0 0 1 0 0 0 0 1 0 0 0 0 1 t P P ),,, ( 3 2 1 0 1 γ γ γ γ γ γ ν ν µ µ = = Γ 1 : : : Γ P P P P x x P ν ν µ µ vector axial vector ν ν µ µ γ γ Γ ν γ
Mott Parity P2 Mott target Mott Parity Parity Γ = 1 0 0 0 0 1 0 0 0 0 1 0 0 0 0 1 t P P ),,, ( 3 2 1 0 1 γ γ γ γ γ γ ν ν µ µ = = Γ 1 : : : Γ P P P P x x P ν ν µ µ vector axial vector ν ν µ µ γ γ Γ ν γ
64 3 g=9.85 m/s 2 g=9.791 m/s 2 36, km ( ) 1 () 2 () m/s : : a) b) kg/m kg/m k
 63 3 Section 3.1 g 3.1 3.1: : 64 3 g=9.85 m/s 2 g=9.791 m/s 2 36, km ( ) 1 () 2 () 3 9.8 m/s 2 3.2 3.2: : a) b) 5 15 4 1 1. 1 3 14. 1 3 kg/m 3 2 3.3 1 3 5.8 1 3 kg/m 3 3 2.65 1 3 kg/m 3 4 6 m 3.1. 65 5
63 3 Section 3.1 g 3.1 3.1: : 64 3 g=9.85 m/s 2 g=9.791 m/s 2 36, km ( ) 1 () 2 () 3 9.8 m/s 2 3.2 3.2: : a) b) 5 15 4 1 1. 1 3 14. 1 3 kg/m 3 2 3.3 1 3 5.8 1 3 kg/m 3 3 2.65 1 3 kg/m 3 4 6 m 3.1. 65 5
Microsoft Word - 章末問題
 1906 R n m 1 = =1 1 R R= 8h ICP s p s HeNeArXe 1 ns 1 1 1 1 1 17 NaCl 1.3 nm 10nm 3s CuAuAg NaCl CaF - - HeNeAr 1.7(b) 2 2 2d = a + a = 2a d = 2a 2 1 1 N = 8 + 6 = 4 8 2 4 4 2a 3 4 π N πr 3 3 4 ρ = = =
1906 R n m 1 = =1 1 R R= 8h ICP s p s HeNeArXe 1 ns 1 1 1 1 1 17 NaCl 1.3 nm 10nm 3s CuAuAg NaCl CaF - - HeNeAr 1.7(b) 2 2 2d = a + a = 2a d = 2a 2 1 1 N = 8 + 6 = 4 8 2 4 4 2a 3 4 π N πr 3 3 4 ρ = = =
30
 3 ............................................2 2...........................................2....................................2.2...................................2.3..............................
3 ............................................2 2...........................................2....................................2.2...................................2.3..............................
. ev=,604k m 3 Debye ɛ 0 kt e λ D = n e n e Ze 4 ln Λ ν ei = 5.6π / ɛ 0 m/ e kt e /3 ν ei v e H + +e H ev Saha x x = 3/ πme kt g i g e n
 003...............................3 Debye................. 3.4................ 3 3 3 3. Larmor Cyclotron... 3 3................ 4 3.3.......... 4 3.3............ 4 3.3...... 4 3.3.3............ 5 3.4.........
003...............................3 Debye................. 3.4................ 3 3 3 3. Larmor Cyclotron... 3 3................ 4 3.3.......... 4 3.3............ 4 3.3...... 4 3.3.3............ 5 3.4.........
 1 1 1 1-1 1 1-9 1-3 1-1 13-17 -3 6-4 6 3 3-1 35 3-37 3-3 38 4 4-1 39 4- Fe C TEM 41 4-3 C TEM 44 4-4 Fe TEM 46 4-5 5 4-6 5 5 51 6 5 1 1-1 1991 1,1 multiwall nanotube 1993 singlewall nanotube ( 1,) sp 7.4eV
1 1 1 1-1 1 1-9 1-3 1-1 13-17 -3 6-4 6 3 3-1 35 3-37 3-3 38 4 4-1 39 4- Fe C TEM 41 4-3 C TEM 44 4-4 Fe TEM 46 4-5 5 4-6 5 5 51 6 5 1 1-1 1991 1,1 multiwall nanotube 1993 singlewall nanotube ( 1,) sp 7.4eV
資料加第2-2-2号表紙.PDF
 2 2 2 RI RI RI --> 82 50 126 20 28 82 8 8 20 28 50 H, He, Li. r C, O, Ne.. U Ne, Fe.. RI RI (RIA GSI OECD = = RI Beam World Wide Radioactive Beam Facilities 3 GANIL GSI RIKEN RIBF NSCL A1200 Notre Dame
2 2 2 RI RI RI --> 82 50 126 20 28 82 8 8 20 28 50 H, He, Li. r C, O, Ne.. U Ne, Fe.. RI RI (RIA GSI OECD = = RI Beam World Wide Radioactive Beam Facilities 3 GANIL GSI RIKEN RIBF NSCL A1200 Notre Dame
128 3 II S 1, S 2 Φ 1, Φ 2 Φ 1 = { B( r) n( r)}ds S 1 Φ 2 = { B( r) n( r)}ds (3.3) S 2 S S 1 +S 2 { B( r) n( r)}ds = 0 (3.4) S 1, S 2 { B( r) n( r)}ds
 127 3 II 3.1 3.1.1 Φ(t) ϕ em = dφ dt (3.1) B( r) Φ = { B( r) n( r)}ds (3.2) S S n( r) Φ 128 3 II S 1, S 2 Φ 1, Φ 2 Φ 1 = { B( r) n( r)}ds S 1 Φ 2 = { B( r) n( r)}ds (3.3) S 2 S S 1 +S 2 { B( r) n( r)}ds
127 3 II 3.1 3.1.1 Φ(t) ϕ em = dφ dt (3.1) B( r) Φ = { B( r) n( r)}ds (3.2) S S n( r) Φ 128 3 II S 1, S 2 Φ 1, Φ 2 Φ 1 = { B( r) n( r)}ds S 1 Φ 2 = { B( r) n( r)}ds (3.3) S 2 S S 1 +S 2 { B( r) n( r)}ds
Gauss Gauss ɛ 0 E ds = Q (1) xy σ (x, y, z) (2) a ρ(x, y, z) = x 2 + y 2 (r, θ, φ) (1) xy A Gauss ɛ 0 E ds = ɛ 0 EA Q = ρa ɛ 0 EA = ρea E = (ρ/ɛ 0 )e
 7 -a 7 -a February 4, 2007 1. 2. 3. 4. 1. 2. 3. 1 Gauss Gauss ɛ 0 E ds = Q (1) xy σ (x, y, z) (2) a ρ(x, y, z) = x 2 + y 2 (r, θ, φ) (1) xy A Gauss ɛ 0 E ds = ɛ 0 EA Q = ρa ɛ 0 EA = ρea E = (ρ/ɛ 0 )e z
7 -a 7 -a February 4, 2007 1. 2. 3. 4. 1. 2. 3. 1 Gauss Gauss ɛ 0 E ds = Q (1) xy σ (x, y, z) (2) a ρ(x, y, z) = x 2 + y 2 (r, θ, φ) (1) xy A Gauss ɛ 0 E ds = ɛ 0 EA Q = ρa ɛ 0 EA = ρea E = (ρ/ɛ 0 )e z
JAJP
 Agilent 7500ce ORS ICP-MS Glenn Woods Agilent Technologies Ltd. 5500 Lakeside, Cheadle Royal Business Park Stockport UK Agilent 7500ce ICP-MS 5 7500ce (ORS) 1 ORS 7500ce ORS ICP-MS ( ) 7500 ICP-MS (27.12
Agilent 7500ce ORS ICP-MS Glenn Woods Agilent Technologies Ltd. 5500 Lakeside, Cheadle Royal Business Park Stockport UK Agilent 7500ce ICP-MS 5 7500ce (ORS) 1 ORS 7500ce ORS ICP-MS ( ) 7500 ICP-MS (27.12
 2 1 7 - TALK ABOUT 21 μ TALK ABOUT 21 Ag As Se 2. 2. 2. Ag As Se 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 Sb Ga Te 2. Sb 2. Ga 2. Te 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 1 2 3 4
2 1 7 - TALK ABOUT 21 μ TALK ABOUT 21 Ag As Se 2. 2. 2. Ag As Se 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 Sb Ga Te 2. Sb 2. Ga 2. Te 1 2 3 4 5 6 7 8 9 1 1 2 3 4 5 6 7 8 9 1 1 2 3 4
1 223 KamLAND 2014 ( 26 ) KamLAND 144 Ce CeLAND 8 Li IsoDAR CeLAND IsoDAR ν e ν µ ν τ ν 1 ν 2 ν MNS m 2 21
 1 3 KamLAND shimizu@awa.tohoku.ac.jp 014 ( 6 ) 1 31 1 KamLAND 144 Ce CeLAND 8 Li IsoDAR CeLAND IsoDAR.1 ν e ν µ ν τ ν 1 ν ν 3 3 3 MNS m 1 = 7.5 10 5 ev m 31 m 3 =.3 10 3 ev 100 m ν e [1] 71 Ga SAGEGallex
1 3 KamLAND shimizu@awa.tohoku.ac.jp 014 ( 6 ) 1 31 1 KamLAND 144 Ce CeLAND 8 Li IsoDAR CeLAND IsoDAR.1 ν e ν µ ν τ ν 1 ν ν 3 3 3 MNS m 1 = 7.5 10 5 ev m 31 m 3 =.3 10 3 ev 100 m ν e [1] 71 Ga SAGEGallex
untitled
 TOF ENMA JAEA-RMS) TOF Pre-scission JAERI-RMS (m-state 16 O + 27 Al 150MeV d TOF Nucl. Phys. A444, 349-364 (1985). l = m d Pre-scission Scission 10-19 (Post_scission) (Pre-scission) Proton_fission Alpha_fission
TOF ENMA JAEA-RMS) TOF Pre-scission JAERI-RMS (m-state 16 O + 27 Al 150MeV d TOF Nucl. Phys. A444, 349-364 (1985). l = m d Pre-scission Scission 10-19 (Post_scission) (Pre-scission) Proton_fission Alpha_fission
Yuzo Nakamura, Kagoshima Univ., Dept Mech Engr. perfect crystal imperfect crystal point defect vacancy self-interstitial atom substitutional impurity
 perfect crystal imperfect crystal point defect vacancy self-interstitial atom substitutional impurity atom interstitial impurity atom line defect dislocation planar defect surface grain boundary interface
perfect crystal imperfect crystal point defect vacancy self-interstitial atom substitutional impurity atom interstitial impurity atom line defect dislocation planar defect surface grain boundary interface
Note.tex 2008/09/19( )
 1 20 9 19 2 1 5 1.1........................ 5 1.2............................. 8 2 9 2.1............................. 9 2.2.............................. 10 3 13 3.1.............................. 13 3.2..................................
1 20 9 19 2 1 5 1.1........................ 5 1.2............................. 8 2 9 2.1............................. 9 2.2.............................. 10 3 13 3.1.............................. 13 3.2..................................
 B 1 B.1.......................... 1 B.1.1................. 1 B.1.2................. 2 B.2........................... 5 B.2.1.......................... 5 B.2.2.................. 6 B.2.3..................
B 1 B.1.......................... 1 B.1.1................. 1 B.1.2................. 2 B.2........................... 5 B.2.1.......................... 5 B.2.2.................. 6 B.2.3..................
 [Ver. 0.2] 1 2 3 4 5 6 7 1 1.1 1.2 1.3 1.4 1.5 1 1.1 1 1.2 1. (elasticity) 2. (plasticity) 3. (strength) 4. 5. (toughness) 6. 1 1.2 1. (elasticity) } 1 1.2 2. (plasticity), 1 1.2 3. (strength) a < b F
[Ver. 0.2] 1 2 3 4 5 6 7 1 1.1 1.2 1.3 1.4 1.5 1 1.1 1 1.2 1. (elasticity) 2. (plasticity) 3. (strength) 4. 5. (toughness) 6. 1 1.2 1. (elasticity) } 1 1.2 2. (plasticity), 1 1.2 3. (strength) a < b F
RAA-05(201604)MRA対応製品ver6
 M R A 対 応 製 品 ISO/IEC 17025 ISO/IEC 17025は 試験所及び校正機関が特定の試験又は 校正を実施する能力があるものとして認定を 受けようとする場合の一般要求事項を規定した国際規格 国際相互承認 MRA Mutual Recognition Arrangement 相互承認協定 とは 試験 検査を実施する試験所 検査機関を認定する国際組織として ILAC 国際試験所認定協力機構
M R A 対 応 製 品 ISO/IEC 17025 ISO/IEC 17025は 試験所及び校正機関が特定の試験又は 校正を実施する能力があるものとして認定を 受けようとする場合の一般要求事項を規定した国際規格 国際相互承認 MRA Mutual Recognition Arrangement 相互承認協定 とは 試験 検査を実施する試験所 検査機関を認定する国際組織として ILAC 国際試験所認定協力機構
修士論文
 SAW 14 2 M3622 i 1 1 1-1 1 1-2 2 1-3 2 2 3 2-1 3 2-2 5 2-3 7 2-3-1 7 2-3-2 2-3-3 SAW 12 3 13 3-1 13 3-2 14 4 SAW 19 4-1 19 4-2 21 4-2-1 21 4-2-2 22 4-3 24 4-4 35 5 SAW 36 5-1 Wedge 36 5-1-1 SAW 36 5-1-2
SAW 14 2 M3622 i 1 1 1-1 1 1-2 2 1-3 2 2 3 2-1 3 2-2 5 2-3 7 2-3-1 7 2-3-2 2-3-3 SAW 12 3 13 3-1 13 3-2 14 4 SAW 19 4-1 19 4-2 21 4-2-1 21 4-2-2 22 4-3 24 4-4 35 5 SAW 36 5-1 Wedge 36 5-1-1 SAW 36 5-1-2
2_R_新技術説明会(佐々木)
 % U: 6.58%, Np, Am:.5%, Pu:.% 5.8% Cs 6.5% Sr %.9%Mo 8.74% Tc.9% TODA C 8 H 7 C 8 H 7 N CH C CH N CH O C C 8 H 7 O N MIDOA C 8 H 7 DOODA NTA + HN(C 8 H 7 ) + H O DCC + SOCl + HN(C 8 H 7 ) + Cl TODA (TODA)
% U: 6.58%, Np, Am:.5%, Pu:.% 5.8% Cs 6.5% Sr %.9%Mo 8.74% Tc.9% TODA C 8 H 7 C 8 H 7 N CH C CH N CH O C C 8 H 7 O N MIDOA C 8 H 7 DOODA NTA + HN(C 8 H 7 ) + H O DCC + SOCl + HN(C 8 H 7 ) + Cl TODA (TODA)
global global mass region (matter ) & (I) M3Y semi-microscopic int. Ref.: H. N., P. R. C68, ( 03) N. P. A722, 117c ( 03) Proc. of NENS03 (to be
 Gogny hard core spin-isospin property @ RCNP (Mar. 22 24, 2004) Collaborator: M. Sato (Chiba U, ) ( ) global global mass region (matter ) & (I) M3Y semi-microscopic int. Ref.: H. N., P. R. C68, 014316
Gogny hard core spin-isospin property @ RCNP (Mar. 22 24, 2004) Collaborator: M. Sato (Chiba U, ) ( ) global global mass region (matter ) & (I) M3Y semi-microscopic int. Ref.: H. N., P. R. C68, 014316
X線分析の進歩36 別刷
 X X X-Ray Fluorescence Analysis on Environmental Standard Reference Materials with a Dry Battery X-Ray Generator Hideshi ISHII, Hiroya MIYAUCHI, Tadashi HIOKI and Jun KAWAI Copyright The Discussion Group
X X X-Ray Fluorescence Analysis on Environmental Standard Reference Materials with a Dry Battery X-Ray Generator Hideshi ISHII, Hiroya MIYAUCHI, Tadashi HIOKI and Jun KAWAI Copyright The Discussion Group
N cos s s cos ψ e e e e 3 3 e e 3 e 3 e
 3 3 5 5 5 3 3 7 5 33 5 33 9 5 8 > e > f U f U u u > u ue u e u ue u ue u e u e u u e u u e u N cos s s cos ψ e e e e 3 3 e e 3 e 3 e 3 > A A > A E A f A A f A [ ] f A A e > > A e[ ] > f A E A < < f ; >
3 3 5 5 5 3 3 7 5 33 5 33 9 5 8 > e > f U f U u u > u ue u e u ue u ue u e u e u u e u u e u N cos s s cos ψ e e e e 3 3 e e 3 e 3 e 3 > A A > A E A f A A f A [ ] f A A e > > A e[ ] > f A E A < < f ; >
2 A B A B A A B Ea 1 51 Ea 1 A B A B B A B B A Ea 2 A B Ea 1 ( )k 1 Ea 1 Ea 2 Arrhenius 53 Ea R T k 1 = χe 1 Ea RT k 2 = χe 2 Ea RT 53 A B A B
 5. A B B A B A B B A A B A B 2 A [A] B [B] 51 v = k[a][b] 51 A B 3 0 273.16 A B A B A B A A [A] 52 v= k[a] 52 A B 55 2 A B A B A A B Ea 1 51 Ea 1 A B A B B A B B A Ea 2 A B Ea 1 ( )k 1 Ea 1 Ea 2 Arrhenius
5. A B B A B A B B A A B A B 2 A [A] B [B] 51 v = k[a][b] 51 A B 3 0 273.16 A B A B A B A A [A] 52 v= k[a] 52 A B 55 2 A B A B A A B Ea 1 51 Ea 1 A B A B B A B B A Ea 2 A B Ea 1 ( )k 1 Ea 1 Ea 2 Arrhenius
1 (Berry,1975) 2-6 p (S πr 2 )p πr 2 p 2πRγ p p = 2γ R (2.5).1-1 : : : : ( ).2 α, β α, β () X S = X X α X β (.1) 1 2
 2005 9/8-11 2 2.2 ( 2-5) γ ( ) γ cos θ 2πr πρhr 2 g h = 2γ cos θ ρgr (2.1) γ = ρgrh (2.2) 2 cos θ θ cos θ = 1 (2.2) γ = 1 ρgrh (2.) 2 2. p p ρgh p ( ) p p = p ρgh (2.) h p p = 2γ r 1 1 (Berry,1975) 2-6
2005 9/8-11 2 2.2 ( 2-5) γ ( ) γ cos θ 2πr πρhr 2 g h = 2γ cos θ ρgr (2.1) γ = ρgrh (2.2) 2 cos θ θ cos θ = 1 (2.2) γ = 1 ρgrh (2.) 2 2. p p ρgh p ( ) p p = p ρgh (2.) h p p = 2γ r 1 1 (Berry,1975) 2-6
Z: Q: R: C: sin 6 5 ζ a, b
 Z: Q: R: C: 3 3 7 4 sin 6 5 ζ 9 6 6............................... 6............................... 6.3......................... 4 7 6 8 8 9 3 33 a, b a bc c b a a b 5 3 5 3 5 5 3 a a a a p > p p p, 3,
Z: Q: R: C: 3 3 7 4 sin 6 5 ζ 9 6 6............................... 6............................... 6.3......................... 4 7 6 8 8 9 3 33 a, b a bc c b a a b 5 3 5 3 5 5 3 a a a a p > p p p, 3,
2011de.dvi
 211 ( 4 2 1. 3 1.1............................... 3 1.2 1- -......................... 13 1.3 2-1 -................... 19 1.4 3- -......................... 29 2. 37 2.1................................ 37
211 ( 4 2 1. 3 1.1............................... 3 1.2 1- -......................... 13 1.3 2-1 -................... 19 1.4 3- -......................... 29 2. 37 2.1................................ 37
(Compton Scattering) Beaming 1 exp [i (k x ωt)] k λ k = 2π/λ ω = 2πν k = ω/c k x ωt ( ω ) k α c, k k x ωt η αβ k α x β diag( + ++) x β = (ct, x) O O x
![(Compton Scattering) Beaming 1 exp [i (k x ωt)] k λ k = 2π/λ ω = 2πν k = ω/c k x ωt ( ω ) k α c, k k x ωt η αβ k α x β diag( + ++) x β = (ct, x) O O x (Compton Scattering) Beaming 1 exp [i (k x ωt)] k λ k = 2π/λ ω = 2πν k = ω/c k x ωt ( ω ) k α c, k k x ωt η αβ k α x β diag( + ++) x β = (ct, x) O O x](/thumbs/104/162595168.jpg) Compton Scattering Beaming exp [i k x ωt] k λ k π/λ ω πν k ω/c k x ωt ω k α c, k k x ωt η αβ k α x β diag + ++ x β ct, x O O x O O v k α k α β, γ k γ k βk, k γ k + βk k γ k k, k γ k + βk 3 k k 4 k 3 k
Compton Scattering Beaming exp [i k x ωt] k λ k π/λ ω πν k ω/c k x ωt ω k α c, k k x ωt η αβ k α x β diag + ++ x β ct, x O O x O O v k α k α β, γ k γ k βk, k γ k + βk k γ k k, k γ k + βk 3 k k 4 k 3 k
v v = v 1 v 2 v 3 (1) R = (R ij ) (2) R (R 1 ) ij = R ji (3) 3 R ij R ik = δ jk (4) i=1 δ ij Kronecker δ ij = { 1 (i = j) 0 (i
 1. 1 1.1 1.1.1 1.1.1.1 v v = v 1 v 2 v 3 (1) R = (R ij ) (2) R (R 1 ) ij = R ji (3) R ij R ik = δ jk (4) δ ij Kronecker δ ij = { 1 (i = j) 0 (i j) (5) 1 1.1. v1.1 2011/04/10 1. 1 2 v i = R ij v j (6) [
1. 1 1.1 1.1.1 1.1.1.1 v v = v 1 v 2 v 3 (1) R = (R ij ) (2) R (R 1 ) ij = R ji (3) R ij R ik = δ jk (4) δ ij Kronecker δ ij = { 1 (i = j) 0 (i j) (5) 1 1.1. v1.1 2011/04/10 1. 1 2 v i = R ij v j (6) [
素粒子物理学2 素粒子物理学序論B 2010年度講義第2回
 素粒子物理学2 素粒子物理学序論B 2010年度講義第2回 =1.055 10 34 J sec =6.582 10 22 MeV sec c = 197.33 10 15 MeV m = c = c =1 1 m p = c(mev m) 938M ev = 197 10 15 (m) 938 =0.2 10 13 (cm) 1 m p = (MeV sec) 938M ev = 6.58
素粒子物理学2 素粒子物理学序論B 2010年度講義第2回 =1.055 10 34 J sec =6.582 10 22 MeV sec c = 197.33 10 15 MeV m = c = c =1 1 m p = c(mev m) 938M ev = 197 10 15 (m) 938 =0.2 10 13 (cm) 1 m p = (MeV sec) 938M ev = 6.58
1. 4cm 16 cm 4cm 20cm 18 cm L λ(x)=ax [kg/m] A x 4cm A 4cm 12 cm h h Y 0 a G 0.38h a b x r(x) x y = 1 h 0.38h G b h X x r(x) 1 S(x) = πr(x) 2 a,b, h,π
![1. 4cm 16 cm 4cm 20cm 18 cm L λ(x)=ax [kg/m] A x 4cm A 4cm 12 cm h h Y 0 a G 0.38h a b x r(x) x y = 1 h 0.38h G b h X x r(x) 1 S(x) = πr(x) 2 a,b, h,π 1. 4cm 16 cm 4cm 20cm 18 cm L λ(x)=ax [kg/m] A x 4cm A 4cm 12 cm h h Y 0 a G 0.38h a b x r(x) x y = 1 h 0.38h G b h X x r(x) 1 S(x) = πr(x) 2 a,b, h,π](/thumbs/101/149329485.jpg) . 4cm 6 cm 4cm cm 8 cm λ()=a [kg/m] A 4cm A 4cm cm h h Y a G.38h a b () y = h.38h G b h X () S() = π() a,b, h,π V = ρ M = ρv G = M h S() 3 d a,b, h 4 G = 5 h a b a b = 6 ω() s v m θ() m v () θ() ω() dθ()
. 4cm 6 cm 4cm cm 8 cm λ()=a [kg/m] A 4cm A 4cm cm h h Y a G.38h a b () y = h.38h G b h X () S() = π() a,b, h,π V = ρ M = ρv G = M h S() 3 d a,b, h 4 G = 5 h a b a b = 6 ω() s v m θ() m v () θ() ω() dθ()
TOP URL 1
 TOP URL http://amonphys.web.fc.com/ 3.............................. 3.............................. 4.3 4................... 5.4........................ 6.5........................ 8.6...........................7
TOP URL http://amonphys.web.fc.com/ 3.............................. 3.............................. 4.3 4................... 5.4........................ 6.5........................ 8.6...........................7
 1-x x µ (+) +z µ ( ) Co 2p 3d µ = µ (+) µ ( ) W. Grange et al., PRB 58, 6298 (1998). 1.0 0.5 0.0 2 1 XMCD 0-1 -2-3x10-3 7.1 7.2 7.7 7.8 8.3 8.4 up E down ρ + (E) ρ (E) H, M µ f + f E F f + f f + f X L
1-x x µ (+) +z µ ( ) Co 2p 3d µ = µ (+) µ ( ) W. Grange et al., PRB 58, 6298 (1998). 1.0 0.5 0.0 2 1 XMCD 0-1 -2-3x10-3 7.1 7.2 7.7 7.8 8.3 8.4 up E down ρ + (E) ρ (E) H, M µ f + f E F f + f f + f X L
4 1 Ampère 4 2 Ampere 31
 4. 2 2 Coulomb 2 2 2 ( ) electricity 2 30 4 1 Ampère 4 2 Ampere 31 NS 2 Fleming 4 3 B I r 4 1 0 1.257 10-2 Gm/A µ 0I B = 2πr 4 1 32 4 4 A A A A 4 4 10 9 1 2 12 13 14 4 1 16 4 1 CH 2 =CH 2 28.0313 28 2
4. 2 2 Coulomb 2 2 2 ( ) electricity 2 30 4 1 Ampère 4 2 Ampere 31 NS 2 Fleming 4 3 B I r 4 1 0 1.257 10-2 Gm/A µ 0I B = 2πr 4 1 32 4 4 A A A A 4 4 10 9 1 2 12 13 14 4 1 16 4 1 CH 2 =CH 2 28.0313 28 2
医系の統計入門第 2 版 サンプルページ この本の定価 判型などは, 以下の URL からご覧いただけます. このサンプルページの内容は, 第 2 版 1 刷発行時のものです.
 医系の統計入門第 2 版 サンプルページ この本の定価 判型などは, 以下の URL からご覧いただけます. http://www.morikita.co.jp/books/mid/009192 このサンプルページの内容は, 第 2 版 1 刷発行時のものです. i 2 t 1. 2. 3 2 3. 6 4. 7 5. n 2 ν 6. 2 7. 2003 ii 2 2013 10 iii 1987
医系の統計入門第 2 版 サンプルページ この本の定価 判型などは, 以下の URL からご覧いただけます. http://www.morikita.co.jp/books/mid/009192 このサンプルページの内容は, 第 2 版 1 刷発行時のものです. i 2 t 1. 2. 3 2 3. 6 4. 7 5. n 2 ν 6. 2 7. 2003 ii 2 2013 10 iii 1987
講義ノート 物性研究 電子版 Vol.3 No.1, (2013 年 T c µ T c Kammerlingh Onnes 77K ρ 5.8µΩcm 4.2K ρ 10 4 µωcm σ 77K ρ 4.2K σ σ = ne 2 τ/m τ 77K
 2 2 T c µ T c 1 1.1 1911 Kammerlingh Onnes 77K ρ 5.8µΩcm 4.2K ρ 1 4 µωcm σ 77K ρ 4.2K σ σ = ne 2 τ/m τ 77K τ 4.2K σ 58 213 email:takada@issp.u-tokyo.ac.jp 1933 Meissner Ochsenfeld λ = 1 5 cm B = χ B =
2 2 T c µ T c 1 1.1 1911 Kammerlingh Onnes 77K ρ 5.8µΩcm 4.2K ρ 1 4 µωcm σ 77K ρ 4.2K σ σ = ne 2 τ/m τ 77K τ 4.2K σ 58 213 email:takada@issp.u-tokyo.ac.jp 1933 Meissner Ochsenfeld λ = 1 5 cm B = χ B =
QMII_10.dvi
 65 1 1.1 1.1.1 1.1 H H () = E (), (1.1) H ν () = E ν () ν (). (1.) () () = δ, (1.3) μ () ν () = δ(μ ν). (1.4) E E ν () E () H 1.1: H α(t) = c (t) () + dνc ν (t) ν (), (1.5) H () () + dν ν () ν () = 1 (1.6)
65 1 1.1 1.1.1 1.1 H H () = E (), (1.1) H ν () = E ν () ν (). (1.) () () = δ, (1.3) μ () ν () = δ(μ ν). (1.4) E E ν () E () H 1.1: H α(t) = c (t) () + dνc ν (t) ν (), (1.5) H () () + dν ν () ν () = 1 (1.6)
nm (T = K, p = kP a (1atm( )), 1bar = 10 5 P a = atm) 1 ( ) m / m
 .1 1nm (T = 73.15K, p = 101.35kP a (1atm( )), 1bar = 10 5 P a = 0.9863atm) 1 ( ).413968 10 3 m 3 1 37. 1/3 3.34.414 10 3 m 3 6.0 10 3 = 3.7 (109 ) 3 (nm) 3 10 6 = 3.7 10 1 (nm) 3 = (3.34nm) 3 ( P = nrt,
.1 1nm (T = 73.15K, p = 101.35kP a (1atm( )), 1bar = 10 5 P a = 0.9863atm) 1 ( ).413968 10 3 m 3 1 37. 1/3 3.34.414 10 3 m 3 6.0 10 3 = 3.7 (109 ) 3 (nm) 3 10 6 = 3.7 10 1 (nm) 3 = (3.34nm) 3 ( P = nrt,
 H+He H+He M > 10 M He He Wolf-Rayet Minit = 13 ~ 100 M, Zinit = 0.0001 ~ 0.02 CNO SN 2007bi (Type Ic) progenitor Minit > 100 M, Zinit = 0.004 WO star? Core collapse Rotating star model Saio, Nomoto, and
H+He H+He M > 10 M He He Wolf-Rayet Minit = 13 ~ 100 M, Zinit = 0.0001 ~ 0.02 CNO SN 2007bi (Type Ic) progenitor Minit > 100 M, Zinit = 0.004 WO star? Core collapse Rotating star model Saio, Nomoto, and
I ( ) 2019
 I ( ) 2019 i 1 I,, III,, 1,,,, III,,,, (1 ) (,,, ), :...,, : NHK... NHK, (YouTube ),!!, manaba http://pen.envr.tsukuba.ac.jp/lec/physics/,, Richard Feynman Lectures on Physics Addison-Wesley,,,, x χ,
I ( ) 2019 i 1 I,, III,, 1,,,, III,,,, (1 ) (,,, ), :...,, : NHK... NHK, (YouTube ),!!, manaba http://pen.envr.tsukuba.ac.jp/lec/physics/,, Richard Feynman Lectures on Physics Addison-Wesley,,,, x χ,
Part () () Γ Part ,
 Contents a 6 6 6 6 6 6 6 7 7. 8.. 8.. 8.3. 8 Part. 9. 9.. 9.. 3. 3.. 3.. 3 4. 5 4.. 5 4.. 9 4.3. 3 Part. 6 5. () 6 5.. () 7 5.. 9 5.3. Γ 3 6. 3 6.. 3 6.. 3 6.3. 33 Part 3. 34 7. 34 7.. 34 7.. 34 8. 35
Contents a 6 6 6 6 6 6 6 7 7. 8.. 8.. 8.3. 8 Part. 9. 9.. 9.. 3. 3.. 3.. 3 4. 5 4.. 5 4.. 9 4.3. 3 Part. 6 5. () 6 5.. () 7 5.. 9 5.3. Γ 3 6. 3 6.. 3 6.. 3 6.3. 33 Part 3. 34 7. 34 7.. 34 7.. 34 8. 35
2008/02/18 08:40-10:10, 12:50-14:20 14:30-16:00, 16:10-17:40,
 008/0/18 08:40-10:10, 1:50-14:0 14:30-16:00, 16:10-17:40, 1pt A 1911 Leiden Heike Kammelingh-Onnes H.Kammelingh Onnes 1907 He 1 4. K H H c T c T H c Hg:40 mt, Pb:80 mt, Sn:30 mt 100 mt I c H c H c H
008/0/18 08:40-10:10, 1:50-14:0 14:30-16:00, 16:10-17:40, 1pt A 1911 Leiden Heike Kammelingh-Onnes H.Kammelingh Onnes 1907 He 1 4. K H H c T c T H c Hg:40 mt, Pb:80 mt, Sn:30 mt 100 mt I c H c H c H
IA
 IA 31 4 11 1 1 4 1.1 Planck.............................. 4 1. Bohr.................................... 5 1.3..................................... 6 8.1................................... 8....................................
IA 31 4 11 1 1 4 1.1 Planck.............................. 4 1. Bohr.................................... 5 1.3..................................... 6 8.1................................... 8....................................
C 3 C-1 Ru 2 x Fe x CrSi A A, A, A, A, A Ru 2 x Fe x CrSi 1) 0.3 x 1.8 2) Ru 2 x Fe x CrSi/Pb BTK P Z 3 x = 1.7 Pb BTK P = ) S.Mizutani, S.Ishid
 C 3 C-1 Ru 2 x Fe x CrSi A A, A, A, A, A Ru 2 x Fe x CrSi 1).3 x 1.8 2) Ru 2 x Fe x CrSi/Pb BTK P Z 3 x = 1.7 Pb BTK P =.52 1) S.Mizutani, S.Ishida, S.Fujii and S.Asano, Mater. Tran. 47(26)25. 2) M.Hiroi,
C 3 C-1 Ru 2 x Fe x CrSi A A, A, A, A, A Ru 2 x Fe x CrSi 1).3 x 1.8 2) Ru 2 x Fe x CrSi/Pb BTK P Z 3 x = 1.7 Pb BTK P =.52 1) S.Mizutani, S.Ishida, S.Fujii and S.Asano, Mater. Tran. 47(26)25. 2) M.Hiroi,
1 2 2 (Dielecrics) Maxwell ( ) D H
 2003.02.13 1 2 2 (Dielecrics) 4 2.1... 4 2.2... 5 2.3... 6 2.4... 6 3 Maxwell ( ) 9 3.1... 9 3.2 D H... 11 3.3... 13 4 14 4.1... 14 4.2... 14 4.3... 17 4.4... 19 5 22 6 THz 24 6.1... 24 6.2... 25 7 26
2003.02.13 1 2 2 (Dielecrics) 4 2.1... 4 2.2... 5 2.3... 6 2.4... 6 3 Maxwell ( ) 9 3.1... 9 3.2 D H... 11 3.3... 13 4 14 4.1... 14 4.2... 14 4.3... 17 4.4... 19 5 22 6 THz 24 6.1... 24 6.2... 25 7 26
 K E N Z U 2012 7 16 HP M. 1 1 4 1.1 3.......................... 4 1.2................................... 4 1.2.1..................................... 4 1.2.2.................................... 5................................
K E N Z U 2012 7 16 HP M. 1 1 4 1.1 3.......................... 4 1.2................................... 4 1.2.1..................................... 4 1.2.2.................................... 5................................
untitled
 1 17 () BAC9ABC6ACB3 1 tan 6 = 3, cos 6 = AB=1 BC=2, AC= 3 2 A BC D 2 BDBD=BA 1 2 ABD BADBDA ABC6 BAD = (18 6 ) / 2 = 6 θ = 18 BAD = 12 () AD AD=BADCAD9 ABD ACD A 1 1 1 1 dsinαsinα = d 3 sin β 3 sin β
1 17 () BAC9ABC6ACB3 1 tan 6 = 3, cos 6 = AB=1 BC=2, AC= 3 2 A BC D 2 BDBD=BA 1 2 ABD BADBDA ABC6 BAD = (18 6 ) / 2 = 6 θ = 18 BAD = 12 () AD AD=BADCAD9 ABD ACD A 1 1 1 1 dsinαsinα = d 3 sin β 3 sin β
( )
 18 10 01 ( ) 1 2018 4 1.1 2018............................... 4 1.2 2018......................... 5 2 2017 7 2.1 2017............................... 7 2.2 2017......................... 8 3 2016 9 3.1 2016...............................
18 10 01 ( ) 1 2018 4 1.1 2018............................... 4 1.2 2018......................... 5 2 2017 7 2.1 2017............................... 7 2.2 2017......................... 8 3 2016 9 3.1 2016...............................
I A A441 : April 15, 2013 Version : 1.1 I Kawahira, Tomoki TA (Shigehiro, Yoshida )
 I013 00-1 : April 15, 013 Version : 1.1 I Kawahira, Tomoki TA (Shigehiro, Yoshida) http://www.math.nagoya-u.ac.jp/~kawahira/courses/13s-tenbou.html pdf * 4 15 4 5 13 e πi = 1 5 0 5 7 3 4 6 3 6 10 6 17
I013 00-1 : April 15, 013 Version : 1.1 I Kawahira, Tomoki TA (Shigehiro, Yoshida) http://www.math.nagoya-u.ac.jp/~kawahira/courses/13s-tenbou.html pdf * 4 15 4 5 13 e πi = 1 5 0 5 7 3 4 6 3 6 10 6 17
 http://www1.doshisha.ac.jp/ bukka/qc.html 1. 107 2. 116 3. 1 119 4. 2 126 5. 132 6. 136 7. 1 140 8. 146 9. 2 150 10. 153 11. 157 12. π Hückel 159 13. 163 A-1. Laguerre 165 A-2. Hermite 167 A-3. 170 A-4.
http://www1.doshisha.ac.jp/ bukka/qc.html 1. 107 2. 116 3. 1 119 4. 2 126 5. 132 6. 136 7. 1 140 8. 146 9. 2 150 10. 153 11. 157 12. π Hückel 159 13. 163 A-1. Laguerre 165 A-2. Hermite 167 A-3. 170 A-4.
II (Percolation) ( 3-4 ) 1. [ ],,,,,,,. 2. [ ],.. 3. [ ],. 4. [ ] [ ] G. Grimmett Percolation Springer-Verlag New-York [ ] 3
![II (Percolation) ( 3-4 ) 1. [ ],,,,,,,. 2. [ ],.. 3. [ ],. 4. [ ] [ ] G. Grimmett Percolation Springer-Verlag New-York [ ] 3 II (Percolation) ( 3-4 ) 1. [ ],,,,,,,. 2. [ ],.. 3. [ ],. 4. [ ] [ ] G. Grimmett Percolation Springer-Verlag New-York [ ] 3](/thumbs/94/121840826.jpg) II (Percolation) 12 9 27 ( 3-4 ) 1 [ ] 2 [ ] 3 [ ] 4 [ ] 1992 5 [ ] G Grimmett Percolation Springer-Verlag New-York 1989 6 [ ] 3 1 3 p H 2 3 2 FKG BK Russo 2 p H = p T (=: p c ) 3 2 Kesten p c =1/2 ( )
II (Percolation) 12 9 27 ( 3-4 ) 1 [ ] 2 [ ] 3 [ ] 4 [ ] 1992 5 [ ] G Grimmett Percolation Springer-Verlag New-York 1989 6 [ ] 3 1 3 p H 2 3 2 FKG BK Russo 2 p H = p T (=: p c ) 3 2 Kesten p c =1/2 ( )
( ) ) ) ) 5) 1 J = σe 2 6) ) 9) 1955 Statistical-Mechanical Theory of Irreversible Processes )
 ( 3 7 4 ) 2 2 ) 8 2 954 2) 955 3) 5) J = σe 2 6) 955 7) 9) 955 Statistical-Mechanical Theory of Irreversible Processes 957 ) 3 4 2 A B H (t) = Ae iωt B(t) = B(ω)e iωt B(ω) = [ Φ R (ω) Φ R () ] iω Φ R (t)
( 3 7 4 ) 2 2 ) 8 2 954 2) 955 3) 5) J = σe 2 6) 955 7) 9) 955 Statistical-Mechanical Theory of Irreversible Processes 957 ) 3 4 2 A B H (t) = Ae iωt B(t) = B(ω)e iωt B(ω) = [ Φ R (ω) Φ R () ] iω Φ R (t)
K 1 mk(
 R&D ATN K 1 mk(0.01 0.05 = ( ) (ITS-90)-59.3467 961.78 (T.J.Seebeck) A(+ T 1 I T 0 B - T 1 T 0 E (Thermoelectromotive force) AB =d E(AB) /dt=a+bt----------------- E(AB) T1 = = + + E( AB) α AB a b ( T0
R&D ATN K 1 mk(0.01 0.05 = ( ) (ITS-90)-59.3467 961.78 (T.J.Seebeck) A(+ T 1 I T 0 B - T 1 T 0 E (Thermoelectromotive force) AB =d E(AB) /dt=a+bt----------------- E(AB) T1 = = + + E( AB) α AB a b ( T0
液晶の物理1:連続体理論(弾性,粘性)
 The Physics of Liquid Crystals P. G. de Gennes and J. Prost (Oxford University Press, 1993) Liquid crystals are beautiful and mysterious; I am fond of them for both reasons. My hope is that some readers
The Physics of Liquid Crystals P. G. de Gennes and J. Prost (Oxford University Press, 1993) Liquid crystals are beautiful and mysterious; I am fond of them for both reasons. My hope is that some readers
) a + b = i + 6 b c = 6i j ) a = 0 b = c = 0 ) â = i + j 0 ˆb = 4) a b = b c = j + ) cos α = cos β = 6) a ˆb = b ĉ = 0 7) a b = 6i j b c = i + 6j + 8)
 4 4 ) a + b = i + 6 b c = 6i j ) a = 0 b = c = 0 ) â = i + j 0 ˆb = 4) a b = b c = j + ) cos α = cos β = 6) a ˆb = b ĉ = 0 7) a b = 6i j b c = i + 6j + 8) a b a b = 6i j 4 b c b c 9) a b = 4 a b) c = 7
4 4 ) a + b = i + 6 b c = 6i j ) a = 0 b = c = 0 ) â = i + j 0 ˆb = 4) a b = b c = j + ) cos α = cos β = 6) a ˆb = b ĉ = 0 7) a b = 6i j b c = i + 6j + 8) a b a b = 6i j 4 b c b c 9) a b = 4 a b) c = 7
Microsoft PowerPoint - H22コロキウム [互換モード]
![Microsoft PowerPoint - H22コロキウム [互換モード] Microsoft PowerPoint - H22コロキウム [互換モード]](/thumbs/77/76338633.jpg) xf. Xd z. 3. v 4. 5. Xd i y co y z z θ α «Œ X «+ co θ «z ªªª ª 5 z ªªª ª 8 Xd Xd q λ f ( q) ρ( ) exp( πiq ) dv λ «uθ «z ªªª ª 6 z ªªª ª 9 Xd Xd z z Xd ª «ªªªª «ªˆ «ªªªªª «~~Xd q Xd«Xd«ª ª ªªª f ( q) ρ(
xf. Xd z. 3. v 4. 5. Xd i y co y z z θ α «Œ X «+ co θ «z ªªª ª 5 z ªªª ª 8 Xd Xd q λ f ( q) ρ( ) exp( πiq ) dv λ «uθ «z ªªª ª 6 z ªªª ª 9 Xd Xd z z Xd ª «ªªªª «ªˆ «ªªªªª «~~Xd q Xd«Xd«ª ª ªªª f ( q) ρ(
C: PC H19 A5 2.BUN Ohm s law
 C: PC H19 A5 2.BUN 19 8 6 3 19 3.1........................... 19 3.2 Ohm s law.................... 21 3.3.......................... 24 4 26 4.1................................. 26 4.2.................................
C: PC H19 A5 2.BUN 19 8 6 3 19 3.1........................... 19 3.2 Ohm s law.................... 21 3.3.......................... 24 4 26 4.1................................. 26 4.2.................................
( )
 7..-8..8.......................................................................... 4.................................... 3...................................... 3..3.................................. 4.3....................................
7..-8..8.......................................................................... 4.................................... 3...................................... 3..3.................................. 4.3....................................
7 π L int = gψ(x)ψ(x)φ(x) + (7.4) [ ] p ψ N = n (7.5) π (π +,π 0,π ) ψ (σ, σ, σ )ψ ( A) σ τ ( L int = gψψφ g N τ ) N π * ) (7.6) π π = (π, π, π ) π ±
![7 π L int = gψ(x)ψ(x)φ(x) + (7.4) [ ] p ψ N = n (7.5) π (π +,π 0,π ) ψ (σ, σ, σ )ψ ( A) σ τ ( L int = gψψφ g N τ ) N π * ) (7.6) π π = (π, π, π ) π ± 7 π L int = gψ(x)ψ(x)φ(x) + (7.4) [ ] p ψ N = n (7.5) π (π +,π 0,π ) ψ (σ, σ, σ )ψ ( A) σ τ ( L int = gψψφ g N τ ) N π * ) (7.6) π π = (π, π, π ) π ±](/thumbs/91/106462075.jpg) 7 7. ( ) SU() SU() 9 ( MeV) p 98.8 π + π 0 n 99.57 9.57 97.4 497.70 δm m 0.4%.% 0.% 0.8% π 9.57 4.96 Σ + Σ 0 Σ 89.6 9.46 K + K 0 49.67 (7.) p p = αp + βn, n n = γp + δn (7.a) [ ] p ψ ψ = Uψ, U = n [ α
7 7. ( ) SU() SU() 9 ( MeV) p 98.8 π + π 0 n 99.57 9.57 97.4 497.70 δm m 0.4%.% 0.% 0.8% π 9.57 4.96 Σ + Σ 0 Σ 89.6 9.46 K + K 0 49.67 (7.) p p = αp + βn, n n = γp + δn (7.a) [ ] p ψ ψ = Uψ, U = n [ α
µµ InGaAs/GaAs PIN InGaAs PbS/PbSe InSb InAs/InSb MCT (HgCdTe)
 1001 µµ 1.... 2 2.... 7 3.... 9 4. InGaAs/GaAs PIN... 10 5. InGaAs... 17 6. PbS/PbSe... 18 7. InSb... 22 8. InAs/InSb... 23 9. MCT (HgCdTe)... 25 10.... 28 11.... 29 12. (Si)... 30 13.... 33 14.... 37
1001 µµ 1.... 2 2.... 7 3.... 9 4. InGaAs/GaAs PIN... 10 5. InGaAs... 17 6. PbS/PbSe... 18 7. InSb... 22 8. InAs/InSb... 23 9. MCT (HgCdTe)... 25 10.... 28 11.... 29 12. (Si)... 30 13.... 33 14.... 37

