Synthesis and Property of the Mn Doped Oxide Red Phosphors in the Calcium Aluminates Koji INOUE, Shinya IWATA and Shinobu HASHIMOTO Recently, the deve
|
|
|
- あきとし あきくぼ
- 5 years ago
- Views:
Transcription
1 Synthesis and Property of the Mn Doped Oxide Red Phosphors in the Calcium Aluminates Koji INOUE, Shinya IWATA and Shinobu HASHIMOTO Recently, the development of new sulfide-free phosphors with high efficiency is urgently needed for VFDs and FEDs. The fabrication of Calcium Aluminate and the effect of partially substituted Mn on the photoluminescence (PL) were investigated in this research. In the samples annealed at 1923 K for 3 h in air, red photoluminescence was observed.the PL material is expected as a low-cost and red light-emitting diodes. Key words : Red Phosphor, Calcium Aluminates, Photoluminescence(PL), VFD, FED, LED 1-7) CA Eu 2+ CaAl2O4 :Eu 2+ 1) Nd 3+ CaAl 2 O 4 :Eu 2+, Nd 3+ 2) CA2 Ce 3+ CaAl4O7 :Ce 3+ 3) CA6 Mn 4+ CaAl12O19 :Mn 4+ 4) Cr 3+ CaAl 12 O 19 :Cr 3+ 5) C3A Eu 3+ Ca 3 Al 2 O 6 :Eu 3+ 6) C12A7 Eu 2+ Ca12Al14O33 :Ce 3+ 7) 1 C12A7 8) CA Eu 2+ Eu 3+ 3) 14) Nd 3+, Dy 3+, Ti 3+, Er 3+, Ho 3+ Li +, Na +, K + 4), 10)-13) 15) Er 3+ 16) Mn 2+ Ce 3+ 17) Ce 3+ CA2 Ce 3+ Tb 3+ 5) Pr 3+ 18) Mn 4+ 6) Mg 2+ 19) CA6 Cr 3+ 7) Eu 2+ 20) Ce 3+ 21) C3A Eu 3+ 8) Eu 2+ Nd 3+ 22) Cl - 23 C12A7 Ce 3+ 9) Er 3+ 24) Au - 25) LED CaAl12O19 :Mn 4+ CaAl 12 O 19 magneto-plumbite structure CaAl 12 O 19
2 ) 1 CaAl12O19 5 Al 3+ Al (1 ) (1 ) 12 Ca 2+ 5 CaAl12O19 :Mn A. Bergstein W.B. White 4)CaAl 12 O 19 CaAl 12 O 19 PL Mn 2+, Mn 3+, Mn 4+ Mn nm Mn nm LED CaAl 12 O 19 : Mn 4+ LED LED GaN Y3Al5O12: Ce 3+ GaN 400 nm LED CaAl 12 O 19 : Mn 4+ Mn nm 500nm LED LED CaAl 12 O 19 : Mn 4+ 9) CaF2 MgF2 Mn 4+ CaAl12O19: Mn 4+ 2 CaAl 12 O 19 : Mn ) Mg 12-13) Mn CaAl 12 O 19 :Mn 4+ Mn Mn Al 0.5 mol% 20 mol% (CaAl12-xO19: Mnxx = ) Mn Mn Mn CaAl 12-x O 19 : Mn x
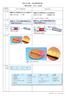
3 1)(CaCO399.99%, 10m) 2)(-Al 2 O %, 5m) 3)()(MnO99.0%, 2-3m) 2 (Electron Spin Resonance ESR) ESR 3 Mn CaAl 12-x O 19 : Mn x (x = 0.000, 0.005, 0.010, 0.020, 0.025, 0.050, 0.100)XRD CaAl12-xO19: Mnx MnO Al2O3 x= K3 200K/h 200K/h X X-ray DiffractionXRD (PhotoluminescencePL) Intensity(a.u.) JCPDS card Diffraction angle 2θ (deg.) 3 CaAl 12 O 19 MnO Al 2 O 3 CaCO K CaO CO2 CaO 1923K CaO Al 2 O 3 Al 2 O 3 4 CaAl 12-x O 19 : Mn x 2= 67
4 XRD Normalized intensity (a.u.) Diffraction angle 2θ (deg.) 4 Mn Ca 2+ = 100 pm, Al 3+ = 53 pm, Mn 4+ = 53 pm, Mn 2+ = 67 pm Mn 2+, Mn 4+ Ca 2+ Mn 2+, Mn 4+ Ca 2+ Al 3+ Mn 2+, Mn 4+, Al 3+ Mn 4+ Al 3+ Mn 2+ Mn 2+ Al 3+ Mn 4+ Al 3+ Mn 4+ Mn 2+ Mn 4+ -Mn 2+ Mn Mn 2+, Mn 4+ Mn 4+ Mn 2+ 5 CaAl12-xO19: Mnx (x = 0.000, 0.005, 0.010, 0.020) PL 6 CaAl 12-x O 19 : Mn x (x =0.020, 0.025, 0.050, 0.100) PL Mn em =655 nm, ex =325 nm 400nm A2 4 T 1, 2 T 2, 4 T 2 325, 385, 462 nm 2 T 1, 2 E 4 A 2 643, 655 nm
5 665 nm 2 E 4 A2 4, 12,13) PL 5 66, 19) Mn E/B 4 T 1 2 T 2 4 T 2 2 T 1 2 E 8 Mn 2 mol%(x = 0.020) Mn 14) Mn x = nm Δ/B 8 Mn 655 nm 4 A 2 9 Mn CaAl 12-x O 19 : Mn x x = 0.000, 0.005, 0.010, 0.020, 0.025, 0.050, 0.100)ESR Intensity (a.u.) x (in CaAl 12-x O 19 : Mn ) x G Fe 3+ 15, 16, x = 0 Fe 3+ x = G Mn ))Mn I = 5/2
6 2I+1 = 6 Mn x = G 6 Mn 4+ 16, 18, 20) 3300 G x = x = 0.005, 0.010, G ESR PL x = G (1)10 Mn Mn x = G 4000 G (1)Mn 4+ 16, 18 x = Mn x = G G Mn 11 x = 0.025, 0.050, ESR Exchange Broadening 39), 45), 46) Mn x = Mn CaAl 12 O 19 Al 3+ CaAl12O19 Mn Mn 2+ [x = ] Mn 4+ (, (1)) [x = ] Mn 4+ (, ) [x = ] 1700 G 3300 G Mn 4+ Mn 4+ 1 Al 3+ Ca 2+ Mn 4+ Ca 2+ Mn x = x = Mn Mn CaAl 12 O 19 Mn Mn 2+ [x = ] Mn 4+ (, (1)) [x = ] Mn 4+ (, ) [x = ]
7 JST 1) F. C. Palilla et al, Eu 2+ and Ce 3+ emission in sulphate based phosphors J.Electrochem. Soc., 115, p642 (1968) 2) T. Matsuzawa et al, Preparation and characterization of an Hf 4+ -doped zinc silicate long-lasting phosphorescent material J. Electrochem. Soc., 143, p2670 (1996) 3) Dongdong Jia et al, Luminescence and energy transfer in CaAl4O7 : Tb 3+, Ce 3+ Wu, J. Lumin., 93, p107 (2001) 4) A. Bergstein et al, Manganese-Activated Luminescence in SrAl12O19 and CaAl12O19 J. Electrochem. Soc., 118, p1166 (1971) 5) Vijay Singh et al, Photoluminescence and EPR studies of Cr-doped hibonite (CaAl 12 O 19 ) phosphors Solid State Sci., 10, p1525 (2008) 6) Xiaorui Gao et al, Preparation and photoluminescence property of a loose powder, Ca 3 Al 2 O 6 :Eu 3+ by calcination of a layered double hydroxide precursor J. Solid State Chem.,181, p1776 (2008) 7) E.Tõldsepet al, Synthesis and luminescence properties of Ce 3+ doped nanoporous12cao 7Al 2 O 3 powders and ceramics Opt. Mat., 32, p784 (2010) 8) Katsuro Hayashi et al, Hopping and optical absorption of electrons in nano-porouscrystal12cao 7Al 2 O 3 Nature, 419, p462 (2002) 9) T. Murata et al, Fluorescencepropertiesof 20) D. Ramirez-Rosales et al, Electron spin Mn 4+ in CaAl12O19 compounds as red-emitingphosphorforwhiteled J. Lumin., 114, p207 (2005) 10) Vijay Singh et al, Luminescence and EPR investigations of Mn activated calcium aluminate prepared via combustion method Opt. Mater., 30, 468 (2007) 11) Vijay Singh et al, Photoluminescence and EPR studies of Cr-doped hibonite (CaAl12O19) phosphors Solid State Sci., 10, p1525 (2008) 12) Y. X. Pan et al, MeltingenthalpyΔHmfor describing glass forming ability of bulk metallic glasses Opt. Lett., 33, p1816 (2003) 13),, (), p (1987) 14) M.G. Brik et al, Spectroscopic and crystal field analysis of absorption and photoluminescence properties of red phosphor CaAl12O19:Mn 4+ modified by MgO J. Alloys Compd., 509, p1452 (2011) 15) D. Cordischi et al, ESR and reflectance spectra of manganese in polycrystalline TiO2 (rutile) J. Solid State Chem., 15, p82 (1975) 16) A. Amorelli et al, Photoluminescence of cubic and monoclinic Gd 2 O 3 :Eu phosphors prepared by flame spray pyrolysis J. Chem. Soc., Faraday Trans., 85, 4031 (1989) 17) Vijay Singh et al, Mn 2+ activated MgSrAl 10 O 17 green-emitting phosphor A luminescence and EPR study J. Lumin., 128, p1474 (2008) 18) R. Arroyo et al, Influence of manganese ions on the anatase rutile phase transition of TiO 2 prepared by the sol gel process Mater. Lett., 54, p397 (2002) 19) Vijay Singh et al, Measurement of the average L-shell fluorescence yields in elements 40<Z<53 Physica B., 403, p120 (2008) resonance study of the conversion of Mn 4+ to Mn 2+ in the Pb1 xeuxti1 ymnyo3 ceramic system Solid State Commun., 118, p371 (2001)
SPring8菅野印刷.PDF
 20021219Spring-8 Ah/kg Ah/dm 3 Li -3.01 540 3860 2090 Na -2.71 970 1160 1140 Al -1.66 2690 2980 8100 Zn -0.76 7140 820 5800 Fe -0.44 7850 960 7500 Cd -0.40 8650 480 4100 Pb -0.13 11340 260 2900 H 2 0
20021219Spring-8 Ah/kg Ah/dm 3 Li -3.01 540 3860 2090 Na -2.71 970 1160 1140 Al -1.66 2690 2980 8100 Zn -0.76 7140 820 5800 Fe -0.44 7850 960 7500 Cd -0.40 8650 480 4100 Pb -0.13 11340 260 2900 H 2 0
untitled
 254nm UV TiO 2 20nm :Sr 5 Ta 4 O 15 3 4 KEY-1 KEY-2 (Ti,Nb,Ta) 5 KEY-1 KEY-2 6 7 NiO/ Sr 2 Ta 2 O 7 mmol h -1 g -1 20 15 10 5 H 2 O 2 H 2 O 2 0 0 2 4 6 8 10 12 NiO/Sr 2 Ta 2 O 7 The synthesis of photocatalysts
254nm UV TiO 2 20nm :Sr 5 Ta 4 O 15 3 4 KEY-1 KEY-2 (Ti,Nb,Ta) 5 KEY-1 KEY-2 6 7 NiO/ Sr 2 Ta 2 O 7 mmol h -1 g -1 20 15 10 5 H 2 O 2 H 2 O 2 0 0 2 4 6 8 10 12 NiO/Sr 2 Ta 2 O 7 The synthesis of photocatalysts
Fig. ph Si-O-Na H O Si- Na OH Si-O-Si OH Si-O Si-OH Si-O-Si Si-O Si-O Si-OH Si-OH Si-O-Si H O 6
 NMR ESR NMR 5 Fig. ph Si-O-Na H O Si- Na OH Si-O-Si OH Si-O Si-OH Si-O-Si Si-O Si-O Si-OH Si-OH Si-O-Si H O 6 Fig. (a) Na O-B -Si Na O-B Si Fig. (b) Na O-CaO-SiO Na O-CaO-B -Si. Na O-. CaO-. Si -. Al O
NMR ESR NMR 5 Fig. ph Si-O-Na H O Si- Na OH Si-O-Si OH Si-O Si-OH Si-O-Si Si-O Si-O Si-OH Si-OH Si-O-Si H O 6 Fig. (a) Na O-B -Si Na O-B Si Fig. (b) Na O-CaO-SiO Na O-CaO-B -Si. Na O-. CaO-. Si -. Al O
000..\..
 Bull. Nagoya Univ. Museum No. 20, 79 91, 2004 Quantitative chemical analysis of rocks with X-ray fluorescence analyzer XRF-1800 NAKAZAKI Mineko TSUBOI Motohiro KANAGAWA Kazuyo KATO Takenori SUZUKI Kazuhiro
Bull. Nagoya Univ. Museum No. 20, 79 91, 2004 Quantitative chemical analysis of rocks with X-ray fluorescence analyzer XRF-1800 NAKAZAKI Mineko TSUBOI Motohiro KANAGAWA Kazuyo KATO Takenori SUZUKI Kazuhiro
PowerPoint プレゼンテーション
 2004 3 3 2 3 4 5 6 7 8 9 10 T. Ito, A. Yamamoto, et al., J. Chem. Soc., Chem. Commun., 136 (1974) J. Chem. Soc., Dalton Trans., 1783 (1974) J. Chem. Soc., Dalton Trans., 1398 (1975) 11 T.Ito, A. Yamamoto,
2004 3 3 2 3 4 5 6 7 8 9 10 T. Ito, A. Yamamoto, et al., J. Chem. Soc., Chem. Commun., 136 (1974) J. Chem. Soc., Dalton Trans., 1783 (1974) J. Chem. Soc., Dalton Trans., 1398 (1975) 11 T.Ito, A. Yamamoto,
36 th IChO : - 3 ( ) , G O O D L U C K final 1
 36 th ICh - - 5 - - : - 3 ( ) - 169 - -, - - - - - - - G D L U C K final 1 1 1.01 2 e 4.00 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 16.00 9 F 19.00 10 Ne 20.18 11 Na 22.99 12 Mg 24.31 Periodic
36 th ICh - - 5 - - : - 3 ( ) - 169 - -, - - - - - - - G D L U C K final 1 1 1.01 2 e 4.00 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 16.00 9 F 19.00 10 Ne 20.18 11 Na 22.99 12 Mg 24.31 Periodic
微粒子合成化学・講義
 http://www.tagen.tohoku.ac.jp/labo/muramatsu/mura/main.html E-mail: mura@tagen.tohoku.ac.jp 1 2 3 1m 10cm 1cm 1mm 100 m 10 m 1 m 100nm 10nm 1nm 1 100 m 10 m 1 m 1nm 100nm 10nm 4 5 6 7 1m 10cm 1cm 1mm 100
http://www.tagen.tohoku.ac.jp/labo/muramatsu/mura/main.html E-mail: mura@tagen.tohoku.ac.jp 1 2 3 1m 10cm 1cm 1mm 100 m 10 m 1 m 100nm 10nm 1nm 1 100 m 10 m 1 m 1nm 100nm 10nm 4 5 6 7 1m 10cm 1cm 1mm 100
03J_sources.key
 Radiation Detection & Measurement (1) (2) (3) (4)1 MeV ( ) 10 9 m 10 7 m 10 10 m < 10 18 m X 10 15 m 10 15 m ......... (isotope)...... (isotone)......... (isobar) 1 1 1 0 1 2 1 2 3 99.985% 0.015% ~0% E
Radiation Detection & Measurement (1) (2) (3) (4)1 MeV ( ) 10 9 m 10 7 m 10 10 m < 10 18 m X 10 15 m 10 15 m ......... (isotope)...... (isotone)......... (isobar) 1 1 1 0 1 2 1 2 3 99.985% 0.015% ~0% E
1 1 H Li Be Na M g B A l C S i N P O S F He N Cl A e K Ca S c T i V C Mn Fe Co Ni Cu Zn Ga Ge As Se B K Rb S Y Z Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb T e
 No. 1 1 1 H Li Be Na M g B A l C S i N P O S F He N Cl A e K Ca S c T i V C Mn Fe Co Ni Cu Zn Ga Ge As Se B K Rb S Y Z Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb T e I X e Cs Ba F Ra Hf Ta W Re Os I Rf Db Sg Bh
No. 1 1 1 H Li Be Na M g B A l C S i N P O S F He N Cl A e K Ca S c T i V C Mn Fe Co Ni Cu Zn Ga Ge As Se B K Rb S Y Z Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb T e I X e Cs Ba F Ra Hf Ta W Re Os I Rf Db Sg Bh
** Department of Materials Science and Engineering, University of California, Los Angeles, CA 90025, USA) Preparation of Magnetopulmbite Type Ferrite
 ** Department of Materials Science and Engineering, University of California, Los Angeles, CA 90025, USA) Preparation of Magnetopulmbite Type Ferrite Thin Films by Dip-Coating Method and Magnetic Properties
** Department of Materials Science and Engineering, University of California, Los Angeles, CA 90025, USA) Preparation of Magnetopulmbite Type Ferrite Thin Films by Dip-Coating Method and Magnetic Properties
Fundamental Study on the SOX Gas Sensor Utilizing Beta-Alumina with Sputtered Praseodymium Oxide Thin Films by Shinya YAO1*, Kenji MIYAGAWA1, Shigeru
 Fundamental Study on the SOX Gas Sensor Utilizing Beta-Alumina with Sputtered Praseodymium Oxide Thin Films by Shinya YAO1*, Kenji MIYAGAWA1, Shigeru IIJIMA2 and Zensaku KOZUKA1 1. Faculty of Engineering,
Fundamental Study on the SOX Gas Sensor Utilizing Beta-Alumina with Sputtered Praseodymium Oxide Thin Films by Shinya YAO1*, Kenji MIYAGAWA1, Shigeru IIJIMA2 and Zensaku KOZUKA1 1. Faculty of Engineering,
2 Zn Zn + MnO 2 () 2 O 2 2 H2 O + O 2 O 2 MnO 2 2 KClO 3 2 KCl + 3 O 2 O 3 or 3 O 2 2 O 3 N 2 () NH 4 NO 2 2 O + N 2 ( ) MnO HCl Mn O + CaCl(ClO
 1 [1]. Zn + 2 H + Zn 2+,. K Ca Na Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Pt Au H (H + ),,. [2] ( ) ( ) CO 2, S, SO 2, NH 3 () + () () + () FeS Fe S ( ) + ( ) ( ) + ( ) 2 NH 4 Cl + Ca(OH) 2 Ca O + 2 NH 3,.,,.,,.,.
1 [1]. Zn + 2 H + Zn 2+,. K Ca Na Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Pt Au H (H + ),,. [2] ( ) ( ) CO 2, S, SO 2, NH 3 () + () () + () FeS Fe S ( ) + ( ) ( ) + ( ) 2 NH 4 Cl + Ca(OH) 2 Ca O + 2 NH 3,.,,.,,.,.
RAA-05(201604)MRA対応製品ver6
 M R A 対 応 製 品 ISO/IEC 17025 ISO/IEC 17025は 試験所及び校正機関が特定の試験又は 校正を実施する能力があるものとして認定を 受けようとする場合の一般要求事項を規定した国際規格 国際相互承認 MRA Mutual Recognition Arrangement 相互承認協定 とは 試験 検査を実施する試験所 検査機関を認定する国際組織として ILAC 国際試験所認定協力機構
M R A 対 応 製 品 ISO/IEC 17025 ISO/IEC 17025は 試験所及び校正機関が特定の試験又は 校正を実施する能力があるものとして認定を 受けようとする場合の一般要求事項を規定した国際規格 国際相互承認 MRA Mutual Recognition Arrangement 相互承認協定 とは 試験 検査を実施する試験所 検査機関を認定する国際組織として ILAC 国際試験所認定協力機構
X線分析の進歩36 別刷
 X X X-Ray Fluorescence Analysis on Environmental Standard Reference Materials with a Dry Battery X-Ray Generator Hideshi ISHII, Hiroya MIYAUCHI, Tadashi HIOKI and Jun KAWAI Copyright The Discussion Group
X X X-Ray Fluorescence Analysis on Environmental Standard Reference Materials with a Dry Battery X-Ray Generator Hideshi ISHII, Hiroya MIYAUCHI, Tadashi HIOKI and Jun KAWAI Copyright The Discussion Group
資源と素材
 (Shigen to Sozai) Vol.116 p. 285 290 (2000) 共存硫酸塩を含む強酸性 MnSO 4 溶液中のオゾン酸化によって生成した沈殿の X 線回折および放電特性 * 西村忠久 1 梅津良昭 2 X-ray Diffraction and Discharge Behavior of Precipitates Produced by Ozone Oxidation in
(Shigen to Sozai) Vol.116 p. 285 290 (2000) 共存硫酸塩を含む強酸性 MnSO 4 溶液中のオゾン酸化によって生成した沈殿の X 線回折および放電特性 * 西村忠久 1 梅津良昭 2 X-ray Diffraction and Discharge Behavior of Precipitates Produced by Ozone Oxidation in
untitled
 NPO 2006( ) 11 14 ( ) (2006/12/3) 1 50% % - - (CO+H2) ( ) 6 44 1) --- 2) ( CO H2 ) 2 3 3 90 3 3 2 3 2004 ( ) 1 1 4 1 20% 5 ( ) ( ) 2 6 MAWERA ) MAWERA ( ) ( ) 7 6MW -- 175kW 8 ( ) 900 10 2 2 2 9 -- - 10
NPO 2006( ) 11 14 ( ) (2006/12/3) 1 50% % - - (CO+H2) ( ) 6 44 1) --- 2) ( CO H2 ) 2 3 3 90 3 3 2 3 2004 ( ) 1 1 4 1 20% 5 ( ) ( ) 2 6 MAWERA ) MAWERA ( ) ( ) 7 6MW -- 175kW 8 ( ) 900 10 2 2 2 9 -- - 10
untitled
 2008-11/13 12 4 12 5 401 501 702 401 501 A-1 9:00-10:30 B-1 9:15-10:30 C-1 9:00-10:30 A-5 9:00-10:30 B-5 9:15-10:30 A A-2 10:45-12:15 B-2 10:45-12:15 C-2 10:45-12:15 A-6 10:45-12:15 B-6 10:45-12:15 A B
2008-11/13 12 4 12 5 401 501 702 401 501 A-1 9:00-10:30 B-1 9:15-10:30 C-1 9:00-10:30 A-5 9:00-10:30 B-5 9:15-10:30 A A-2 10:45-12:15 B-2 10:45-12:15 C-2 10:45-12:15 A-6 10:45-12:15 B-6 10:45-12:15 A B
The Phase Behavior of Monooleoylglycerol-Water Systems Mivoshi Oil & Fat Co.. Ltd. Faculty of Science and Technology, Science University of Tokyo Inst
 The Phase Behavior of Monooleoylglycerol-Water Systems Mivoshi Oil & Fat Co.. Ltd. Faculty of Science and Technology, Science University of Tokyo Institute of Colloid and Interface Science, Science University
The Phase Behavior of Monooleoylglycerol-Water Systems Mivoshi Oil & Fat Co.. Ltd. Faculty of Science and Technology, Science University of Tokyo Institute of Colloid and Interface Science, Science University
42 3 u = (37) MeV/c 2 (3.4) [1] u amu m p m n [1] m H [2] m p = (4) MeV/c 2 = (13) u m n = (4) MeV/c 2 =
![42 3 u = (37) MeV/c 2 (3.4) [1] u amu m p m n [1] m H [2] m p = (4) MeV/c 2 = (13) u m n = (4) MeV/c 2 = 42 3 u = (37) MeV/c 2 (3.4) [1] u amu m p m n [1] m H [2] m p = (4) MeV/c 2 = (13) u m n = (4) MeV/c 2 =](/thumbs/73/68712188.jpg) 3 3.1 3.1.1 kg m s J = kg m 2 s 2 MeV MeV [1] 1MeV=1 6 ev = 1.62 176 462 (63) 1 13 J (3.1) [1] 1MeV/c 2 =1.782 661 731 (7) 1 3 kg (3.2) c =1 MeV (atomic mass unit) 12 C u = 1 12 M(12 C) (3.3) 41 42 3 u
3 3.1 3.1.1 kg m s J = kg m 2 s 2 MeV MeV [1] 1MeV=1 6 ev = 1.62 176 462 (63) 1 13 J (3.1) [1] 1MeV/c 2 =1.782 661 731 (7) 1 3 kg (3.2) c =1 MeV (atomic mass unit) 12 C u = 1 12 M(12 C) (3.3) 41 42 3 u
E-2 A, B, C A, A, B, A, C m-cresol (NEAT) Rh S m-cresol m-cresol m-cresol x x x ,Rh N N N N H H n Polyaniline emeraldine base E-3 II
 E 7, 8, 9 E-1 A, B B A, A, A,, [Novoselov, et al., Science 306, 666, (2004)].,,,.,,,.,. (t a, t b, t c ), [PRB.74, 033413, (2006)],.,, t b /t a t c /t a 0.5., [Ni, etal., ACS, Nano2, 2301, (2008)],, [Zhou
E 7, 8, 9 E-1 A, B B A, A, A,, [Novoselov, et al., Science 306, 666, (2004)].,,,.,,,.,. (t a, t b, t c ), [PRB.74, 033413, (2006)],.,, t b /t a t c /t a 0.5., [Ni, etal., ACS, Nano2, 2301, (2008)],, [Zhou
CH 2 CH CH 2 CH CH 2 CH CH 2 CH 2 COONa CH 2 N CH 2 COONa O Co 2+ O CO CH 2 CH N 2 CH 2 CO 9 Change in Ionic Form of IDA resin with h ph CH 2 NH + COO
 CH 2 CH CH 2 CH CH 2 CH CH 2 CH 2 COONa CH 2 N CH 2 COONa O Co 2+ O CO CH 2 CH N 2 CH 2 CO 9 Change in Ionic Form of IDA resin with h ph CH 2 NH + COOH COOH COOH COO - CH 2 NH + + CH 2 NH COO - COO - COO
CH 2 CH CH 2 CH CH 2 CH CH 2 CH 2 COONa CH 2 N CH 2 COONa O Co 2+ O CO CH 2 CH N 2 CH 2 CO 9 Change in Ionic Form of IDA resin with h ph CH 2 NH + COOH COOH COOH COO - CH 2 NH + + CH 2 NH COO - COO - COO
PowerPoint プレゼンテーション
 2004 SPring-8 2004/6/21 CMOS 2004 2007 2010 2013 nm 90 65 45 32 (nm) 1.2 0.9 0.7 0.6 High-performance Logic Technology Requirements (ITRS 2003) 10 Photoelectron Intensity (arb.units) CTR a-sio2 0.1 HfO
2004 SPring-8 2004/6/21 CMOS 2004 2007 2010 2013 nm 90 65 45 32 (nm) 1.2 0.9 0.7 0.6 High-performance Logic Technology Requirements (ITRS 2003) 10 Photoelectron Intensity (arb.units) CTR a-sio2 0.1 HfO
強相関電子系ペロブスカイト遷移金属酸化物による光エレクトロニクス 平成 12 年 11 月 ~ 平成 18 年 3 月 研究代表者 : 花村榮一 ( 千歳科学技術大学光科学部 教授 )
 強相関電子系ペロブスカイト遷移金属酸化物による光エレクトロニクス 平成 12 年 11 月 ~ 平成 18 年 3 月 研究代表者 : 花村榮一 ( 千歳科学技術大学光科学部 教授 ) χ αβγω χ αβγωβγ αχ χ -1 - ω ω Γ ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω -2 - -3 - BO 2 (Rutile) TiO 2
強相関電子系ペロブスカイト遷移金属酸化物による光エレクトロニクス 平成 12 年 11 月 ~ 平成 18 年 3 月 研究代表者 : 花村榮一 ( 千歳科学技術大学光科学部 教授 ) χ αβγω χ αβγωβγ αχ χ -1 - ω ω Γ ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω ω -2 - -3 - BO 2 (Rutile) TiO 2
1/120 別表第 1(6 8 及び10 関係 ) 放射性物質の種類が明らかで かつ 一種類である場合の放射線業務従事者の呼吸する空気中の放射性物質の濃度限度等 添付 第一欄第二欄第三欄第四欄第五欄第六欄 放射性物質の種類 吸入摂取した 経口摂取した 放射線業 周辺監視 周辺監視 場合の実効線 場合
 1/120 別表第 1(6 8 及び10 関係 ) 放射性物質の種類が明らかで かつ 一種類である場合の放射線業務従事者の呼吸する空気中の放射性物質の濃度限度等 添付 第一欄第二欄第三欄第四欄第五欄第六欄 放射性物質の種類 吸入摂取した 経口摂取した 放射線業 周辺監視 周辺監視 場合の実効線 場合の実効線 務従事者 区域外の 区域外の 量係数 量係数 の呼吸す 空気中の 水中の濃 る空気中 濃度限度
1/120 別表第 1(6 8 及び10 関係 ) 放射性物質の種類が明らかで かつ 一種類である場合の放射線業務従事者の呼吸する空気中の放射性物質の濃度限度等 添付 第一欄第二欄第三欄第四欄第五欄第六欄 放射性物質の種類 吸入摂取した 経口摂取した 放射線業 周辺監視 周辺監視 場合の実効線 場合の実効線 務従事者 区域外の 区域外の 量係数 量係数 の呼吸す 空気中の 水中の濃 る空気中 濃度限度
 BUNSEKI KAGAKU Vol. 34 (1985) Fig. 1 Diagram of anti-stokes delayed fluorescence (ASDF) by the mixed triplet mechanism (the numbered transitions refer to the equations) BUNSEKI KAGAKU Vol. 34 (1985) Fig.
BUNSEKI KAGAKU Vol. 34 (1985) Fig. 1 Diagram of anti-stokes delayed fluorescence (ASDF) by the mixed triplet mechanism (the numbered transitions refer to the equations) BUNSEKI KAGAKU Vol. 34 (1985) Fig.
01-表紙.ai
 B 0 5 0-5 双極子核 I=1/ 2 四極子核 I 1 e Li Be B C N F Ne Na Mg 黒字はNMR 観測不可 Al Si P S Cl Ar K Ca Sc Ti V Cr MnFe Co Ni Cu ZnGaGe As Se Br Kr RbSr Y Zr NbMoTc RuRhPdAgCd In Sn SbTe I Xe Cs Ba La f Ta W Res Ir Pt
B 0 5 0-5 双極子核 I=1/ 2 四極子核 I 1 e Li Be B C N F Ne Na Mg 黒字はNMR 観測不可 Al Si P S Cl Ar K Ca Sc Ti V Cr MnFe Co Ni Cu ZnGaGe As Se Br Kr RbSr Y Zr NbMoTc RuRhPdAgCd In Sn SbTe I Xe Cs Ba La f Ta W Res Ir Pt
 Influence of Material and Thickness of the Specimen to Stress Separation of an Infrared Stress Image Kenji MACHIDA The thickness dependency of the temperature image obtained by an infrared thermography
Influence of Material and Thickness of the Specimen to Stress Separation of an Infrared Stress Image Kenji MACHIDA The thickness dependency of the temperature image obtained by an infrared thermography
元素分析
 : このマークが付してある著作物は 第三者が有する著作物ですので 同著作物の再使用 同著作物の二次的著作物の創作等については 著作権者より直接使用許諾を得る必要があります (PET) 1 18 1 18 H 2 13 14 15 16 17 He 1 2 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Na Mg 3 4 5 6 7 8 9 10 11 12 Al Si P
: このマークが付してある著作物は 第三者が有する著作物ですので 同著作物の再使用 同著作物の二次的著作物の創作等については 著作権者より直接使用許諾を得る必要があります (PET) 1 18 1 18 H 2 13 14 15 16 17 He 1 2 Li Be B C N O F Ne 3 4 5 6 7 8 9 10 Na Mg 3 4 5 6 7 8 9 10 11 12 Al Si P
2_R_新技術説明会(佐々木)
 % U: 6.58%, Np, Am:.5%, Pu:.% 5.8% Cs 6.5% Sr %.9%Mo 8.74% Tc.9% TODA C 8 H 7 C 8 H 7 N CH C CH N CH O C C 8 H 7 O N MIDOA C 8 H 7 DOODA NTA + HN(C 8 H 7 ) + H O DCC + SOCl + HN(C 8 H 7 ) + Cl TODA (TODA)
% U: 6.58%, Np, Am:.5%, Pu:.% 5.8% Cs 6.5% Sr %.9%Mo 8.74% Tc.9% TODA C 8 H 7 C 8 H 7 N CH C CH N CH O C C 8 H 7 O N MIDOA C 8 H 7 DOODA NTA + HN(C 8 H 7 ) + H O DCC + SOCl + HN(C 8 H 7 ) + Cl TODA (TODA)
 1-x x µ (+) +z µ ( ) Co 2p 3d µ = µ (+) µ ( ) W. Grange et al., PRB 58, 6298 (1998). 1.0 0.5 0.0 2 1 XMCD 0-1 -2-3x10-3 7.1 7.2 7.7 7.8 8.3 8.4 up E down ρ + (E) ρ (E) H, M µ f + f E F f + f f + f X L
1-x x µ (+) +z µ ( ) Co 2p 3d µ = µ (+) µ ( ) W. Grange et al., PRB 58, 6298 (1998). 1.0 0.5 0.0 2 1 XMCD 0-1 -2-3x10-3 7.1 7.2 7.7 7.8 8.3 8.4 up E down ρ + (E) ρ (E) H, M µ f + f E F f + f f + f X L
4 1 Ampère 4 2 Ampere 31
 4. 2 2 Coulomb 2 2 2 ( ) electricity 2 30 4 1 Ampère 4 2 Ampere 31 NS 2 Fleming 4 3 B I r 4 1 0 1.257 10-2 Gm/A µ 0I B = 2πr 4 1 32 4 4 A A A A 4 4 10 9 1 2 12 13 14 4 1 16 4 1 CH 2 =CH 2 28.0313 28 2
4. 2 2 Coulomb 2 2 2 ( ) electricity 2 30 4 1 Ampère 4 2 Ampere 31 NS 2 Fleming 4 3 B I r 4 1 0 1.257 10-2 Gm/A µ 0I B = 2πr 4 1 32 4 4 A A A A 4 4 10 9 1 2 12 13 14 4 1 16 4 1 CH 2 =CH 2 28.0313 28 2
H1-H4
 42 S H He Li H He Li Be B C N O F Ne Be B C N O F Ne H He Li Be B H H e L i Na Mg Al Si S Cl Ar Na Mg Al Si S Cl Ar C N O F Ne Na Be B C N O F Ne Na K Sc T i V C r K Sc Ti V Cr M n F e C o N i Mn Fe Mg
42 S H He Li H He Li Be B C N O F Ne Be B C N O F Ne H He Li Be B H H e L i Na Mg Al Si S Cl Ar Na Mg Al Si S Cl Ar C N O F Ne Na Be B C N O F Ne Na K Sc T i V C r K Sc Ti V Cr M n F e C o N i Mn Fe Mg
Fig.2 Optical-microscope image of the Y face-cross sec- tion of the bulk domain structure of a 0.4-mm-thick MgO-LiNbO3 crystal after chemical etching.
 Blue EGreen Solid State Lasers Using MgO-LiNbO3 Periodic Domain Inverted Bulk Crystal and Their Applications Koji KAMIYAMA, Yoji OKAZAKI, and Akinori HARADA Fuji Photo Film. Co., Ltd., Miyanodai Technology
Blue EGreen Solid State Lasers Using MgO-LiNbO3 Periodic Domain Inverted Bulk Crystal and Their Applications Koji KAMIYAMA, Yoji OKAZAKI, and Akinori HARADA Fuji Photo Film. Co., Ltd., Miyanodai Technology
C 3 C-1 Ru 2 x Fe x CrSi A A, A, A, A, A Ru 2 x Fe x CrSi 1) 0.3 x 1.8 2) Ru 2 x Fe x CrSi/Pb BTK P Z 3 x = 1.7 Pb BTK P = ) S.Mizutani, S.Ishid
 C 3 C-1 Ru 2 x Fe x CrSi A A, A, A, A, A Ru 2 x Fe x CrSi 1).3 x 1.8 2) Ru 2 x Fe x CrSi/Pb BTK P Z 3 x = 1.7 Pb BTK P =.52 1) S.Mizutani, S.Ishida, S.Fujii and S.Asano, Mater. Tran. 47(26)25. 2) M.Hiroi,
C 3 C-1 Ru 2 x Fe x CrSi A A, A, A, A, A Ru 2 x Fe x CrSi 1).3 x 1.8 2) Ru 2 x Fe x CrSi/Pb BTK P Z 3 x = 1.7 Pb BTK P =.52 1) S.Mizutani, S.Ishida, S.Fujii and S.Asano, Mater. Tran. 47(26)25. 2) M.Hiroi,
*1 *2 *1 JIS A X TEM 950 TEM JIS Development and Research of the Equipment for Conversion to Harmless Substances and Recycle of Asbe
 *1 *2 *1 JIS A 14812008X TEM 950 TEM 1 2 3 4 JIS Development and Research of the Equipment for Conversion to Harmless Substances and Recycle of Asbestos with Superheated Steam Part 3 An evaluation with
*1 *2 *1 JIS A 14812008X TEM 950 TEM 1 2 3 4 JIS Development and Research of the Equipment for Conversion to Harmless Substances and Recycle of Asbestos with Superheated Steam Part 3 An evaluation with
 MP-AES ICP-QQQ Agilent 5100 ICP-OES Agilent 5100 (SVDV) ICP-OES (DSC) 1 5100 SVDV ICP-OES VistaChip II CCD Agilent 7900 ICP-MS 7700 / 10 7900 ICP-MS ICP-MS FTIR Agilent 7900 ICP-MS Agilent Cary 7000 (UMS)
MP-AES ICP-QQQ Agilent 5100 ICP-OES Agilent 5100 (SVDV) ICP-OES (DSC) 1 5100 SVDV ICP-OES VistaChip II CCD Agilent 7900 ICP-MS 7700 / 10 7900 ICP-MS ICP-MS FTIR Agilent 7900 ICP-MS Agilent Cary 7000 (UMS)
untitled
 E-mail: khatano@fms.saitama-u.ac.jp Tel & Fax: 048-858-3535 Toxin Virus Bacterium Glycolipid Glycoprotein Vero toxin (Stx1 and Stx2) Side view Bottom view H H H H N H Grobotriaosyl Ceramide (Gb 3 : Galα1-4Galβ1-4Glcβ-Cer)
E-mail: khatano@fms.saitama-u.ac.jp Tel & Fax: 048-858-3535 Toxin Virus Bacterium Glycolipid Glycoprotein Vero toxin (Stx1 and Stx2) Side view Bottom view H H H H N H Grobotriaosyl Ceramide (Gb 3 : Galα1-4Galβ1-4Glcβ-Cer)
温泉の化学 1
 H O 1,003 516 149 124 2,237 1974 90 110 1km 2,400 ( 100 Mg 200 (98 ) 43,665 mg 38,695 mg 19,000 mg 2000 2000 Na-Ca-Cl 806 1970 1989 10 1991 4 ph 1 981 10,000 1993... (^^; (SO_4^{2-}) " " 1973-1987 1970
H O 1,003 516 149 124 2,237 1974 90 110 1km 2,400 ( 100 Mg 200 (98 ) 43,665 mg 38,695 mg 19,000 mg 2000 2000 Na-Ca-Cl 806 1970 1989 10 1991 4 ph 1 981 10,000 1993... (^^; (SO_4^{2-}) " " 1973-1987 1970
Vol. 21, No. 2 (2014) W 3 mm SUS304 Ni 650 HV 810 HV Ni Ni Table1 Ni Ni μm SUS mm w 50 mm l 3 mm t 2.2 Fig. 1 XY Fig. 3 Sch
 110 : 565-0871 2-1 567-0871 11-1 660-0811 1-9 - 1 tanigawa@jwri.osaka - u.ac.jp Influence of Laser Beam Profile on Cladding Layer TANIGAWA Daichi, ABE Nobuyuki, TSUKAMOTO Masahiro, HAYASHI Yoshihiko, YAMAZAKI
110 : 565-0871 2-1 567-0871 11-1 660-0811 1-9 - 1 tanigawa@jwri.osaka - u.ac.jp Influence of Laser Beam Profile on Cladding Layer TANIGAWA Daichi, ABE Nobuyuki, TSUKAMOTO Masahiro, HAYASHI Yoshihiko, YAMAZAKI
RN201602_cs5_0122.indd
 ISSN 1349-1229 No.416 February 2016 2 SPECIAL TOPIC113 SPECIAL TOPIC 113 FACE Mykinso 113 SPECIAL TOPIC IUPAC 11320151231 RI RIBFRILAC 20039Zn30 Bi83 20047113 20054201283 113 1133 Bh107 20082009 113 113
ISSN 1349-1229 No.416 February 2016 2 SPECIAL TOPIC113 SPECIAL TOPIC 113 FACE Mykinso 113 SPECIAL TOPIC IUPAC 11320151231 RI RIBFRILAC 20039Zn30 Bi83 20047113 20054201283 113 1133 Bh107 20082009 113 113
Fig. la PL spectra of PSL prepared on Si specimen (p = 1 k Q m) with electrochemical etching in HF solution (26wt %) under galvanostatic conditions of
 Vol.47,No.11,1996 Influence of Electrochemical Etching Current Density on Porous Si Luminescence Properties and Microstructure Kazuhiro SHIGYO *, Masahiro SEO *, Kazuhlsa AZUMI * and Hideaki TAKAHASHI
Vol.47,No.11,1996 Influence of Electrochemical Etching Current Density on Porous Si Luminescence Properties and Microstructure Kazuhiro SHIGYO *, Masahiro SEO *, Kazuhlsa AZUMI * and Hideaki TAKAHASHI
Terahertz Color Scanner Takeshi YASUI Terahertz THz spectroscopic imaging is an interesting new tool for nondestructive testing, security screening, b
 Terahertz Color Scanner Takeshi YASUI Terahertz THz spectroscopic imaging is an interesting new tool for nondestructive testing, security screening, biological imaging, and other applications because of
Terahertz Color Scanner Takeshi YASUI Terahertz THz spectroscopic imaging is an interesting new tool for nondestructive testing, security screening, biological imaging, and other applications because of
I A-9 45 A,B, A,B, A,B, A,B, C, A, A, A A, B, C I A A,B, A,B, B,C, A, A A, B, C ( ) I A-11 LuFe 2 O 4 53, A,, B, C,, A, B, C I A-12 I A-13 Lumin
 23 12 7 ( ) I A, I B I A 10:00 10:05 10F L 10:05 11:45 10F L 12:45 14:45 10F S10A I A-1 DC AC Bloch Zener 341 21, JST CREST I A-2 (6,5) 17,,,, A, B, A, B I A-3 X 21, A,, A I A-4 25 A, A, A,B, A A, B JST
23 12 7 ( ) I A, I B I A 10:00 10:05 10F L 10:05 11:45 10F L 12:45 14:45 10F S10A I A-1 DC AC Bloch Zener 341 21, JST CREST I A-2 (6,5) 17,,,, A, B, A, B I A-3 X 21, A,, A I A-4 25 A, A, A,B, A A, B JST
研究成果報告書(基金分)
 (DMS) 1 3 DMS 2 4 DMS II-VI CdTe ZnTe 2 4 DMS 2 4 DMS II-VI 2 3d 4 3d (MBE) II-VI ZnTe CdTe 3d Mn, Cr, Fe 2 4 DMS MBE X (XRD)X (XAFS) II (SQUID) (a) 4 DMS (Cd,Mn,Cr)Te 4 DMS II-VI CdTe 2 Mn, Cr (Cd,Mn,Cr)Te
(DMS) 1 3 DMS 2 4 DMS II-VI CdTe ZnTe 2 4 DMS 2 4 DMS II-VI 2 3d 4 3d (MBE) II-VI ZnTe CdTe 3d Mn, Cr, Fe 2 4 DMS MBE X (XRD)X (XAFS) II (SQUID) (a) 4 DMS (Cd,Mn,Cr)Te 4 DMS II-VI CdTe 2 Mn, Cr (Cd,Mn,Cr)Te
製紙用填料及び顔料の熱分解挙動.PDF
 Thermogravimetric analysis of pigments and fillers for papermaking Toshiharu Enomae Graduate School of Agricultural and Life Sciences Contact e-mail address: enomae@psl.fp.a.u-tokyo.ac.jp A2 350 450 A2
Thermogravimetric analysis of pigments and fillers for papermaking Toshiharu Enomae Graduate School of Agricultural and Life Sciences Contact e-mail address: enomae@psl.fp.a.u-tokyo.ac.jp A2 350 450 A2
X X 1. 1 X 2 X 195 3, 4 Ungár modified Williamson-Hall/Warren-Averbach 5-7 modified modified Rietveld Convolutional Multiple Whole Profile CMWP 8 CMWP
 X X a a b b c Characterization of dislocation evolution during work hardening of stainless steels by using XRD line-profile analysis Tomoaki KATO a, Shigeo SATO a, Yoichi SAITO b, Hidekazu TODOROKI b and
X X a a b b c Characterization of dislocation evolution during work hardening of stainless steels by using XRD line-profile analysis Tomoaki KATO a, Shigeo SATO a, Yoichi SAITO b, Hidekazu TODOROKI b and
Table 1. Chemical composition of Fe203 and SrCO3 used for experiment. Fig. 1. Process of preparion of the specimen.
 Influence of Addition of Some Oxides on the Magnetic Properties of the Strontium Ferrite Magnets Takeshi Anbo, Takahiro Motone, Takashi Furuya and Koichi Sasaki Synopsis Recently high coercivity ferrite
Influence of Addition of Some Oxides on the Magnetic Properties of the Strontium Ferrite Magnets Takeshi Anbo, Takahiro Motone, Takashi Furuya and Koichi Sasaki Synopsis Recently high coercivity ferrite
1
 4 Nano Device Technologies From New Functions of Extreme Substances to Telecommunication Technologies 4-1 Controlling Intermolecular Interactions using Nano- Structural Molecules OTOMO Akira, YOKOYAMA
4 Nano Device Technologies From New Functions of Extreme Substances to Telecommunication Technologies 4-1 Controlling Intermolecular Interactions using Nano- Structural Molecules OTOMO Akira, YOKOYAMA
ナノ粒子のサイズ・形態制御と 構造敏感型触媒プロセスへの応用
 2 3 10 9 m = 1 nm m 4 5 100 m 1m 10cm 100 m 1cm 1mm 10 m 1 m 100nm 10nm 1nm 1 10 m 1 m 1nm 100nm 10nm 10 8 6 1 nm 10 8 7 9 10 11 12 13 14 15 17 18 19 20 21 nm Ni-Zn 2- Ni-Zn Zn Ni Ni-Zn B 5 10 nm 23
2 3 10 9 m = 1 nm m 4 5 100 m 1m 10cm 100 m 1cm 1mm 10 m 1 m 100nm 10nm 1nm 1 10 m 1 m 1nm 100nm 10nm 10 8 6 1 nm 10 8 7 9 10 11 12 13 14 15 17 18 19 20 21 nm Ni-Zn 2- Ni-Zn Zn Ni Ni-Zn B 5 10 nm 23
apple ヲ0 09 apple ヲ apple0309apple076 56ヲ fl 0603apple6ヲ
 1305ィャ00010204ィヲ00ィヲ07ィ ィケ ィ 0500090502ィヲ00ィヲ07ィ ィケ, 2009, 6ァ8 6, 06. 1ィC18 ィャ0501 551.23/21;558.42 07ィ 05ィコィヲ0700ィヲ07ィ 02ィ ィケ ィェ00ィコ020200ィイ040107 ィ 05ィェィヲィョ04ィ 01ィヲ05 05ィャ0001020402 02ィャィェ04ィヲ050501ィ
1305ィャ00010204ィヲ00ィヲ07ィ ィケ ィ 0500090502ィヲ00ィヲ07ィ ィケ, 2009, 6ァ8 6, 06. 1ィC18 ィャ0501 551.23/21;558.42 07ィ 05ィコィヲ0700ィヲ07ィ 02ィ ィケ ィェ00ィコ020200ィイ040107 ィ 05ィェィヲィョ04ィ 01ィヲ05 05ィャ0001020402 02ィャィェ04ィヲ050501ィ
δδ 1 2 δ δ δ δ μ H 2.1 C 2.5 N 3.0 O 3.5 Cl 3.0 S μ
 1961. (Received January 15, 2013) 1) 1. 2. ph 2) 3) 2) 2) 2001 4 6) 7) ph ph ph 1961 1962 1. 2 1 1 δδ 1 2 δ δ δ δ μ 1 1 1 1. H 2.1 C 2.5 N 3.0 O 3.5 Cl 3.0 S 2.5 1 1. μ 1 3. 1 2. 1963 1,4- δ δ 1 3 2 δ
1961. (Received January 15, 2013) 1) 1. 2. ph 2) 3) 2) 2) 2001 4 6) 7) ph ph ph 1961 1962 1. 2 1 1 δδ 1 2 δ δ δ δ μ 1 1 1 1. H 2.1 C 2.5 N 3.0 O 3.5 Cl 3.0 S 2.5 1 1. μ 1 3. 1 2. 1963 1,4- δ δ 1 3 2 δ
表紙
 Akira Fujishima 2005.Vo.3 1 Kiyoshi kanamura kanamura-kiyoshi@c.metro-u.ac.jp 2 2005.Vol.3 Rechargeable Lithium Ion-Battery Active Material Liquid Electrolyte σ = 10-2 10-3 S cm -1 3D Interface of Solid
Akira Fujishima 2005.Vo.3 1 Kiyoshi kanamura kanamura-kiyoshi@c.metro-u.ac.jp 2 2005.Vol.3 Rechargeable Lithium Ion-Battery Active Material Liquid Electrolyte σ = 10-2 10-3 S cm -1 3D Interface of Solid
日立金属技報 Vol.34
 Influence of Misorientation Angle between Adjacent Grains on Magnetization Reversal in Nd-Fe-B Sintered Magnet Tomohito Maki Rintaro Ishii Mitsutoshi Natsumeda Takeshi Nishiuchi Ryo Uchikoshi Masaaki Takezawa
Influence of Misorientation Angle between Adjacent Grains on Magnetization Reversal in Nd-Fe-B Sintered Magnet Tomohito Maki Rintaro Ishii Mitsutoshi Natsumeda Takeshi Nishiuchi Ryo Uchikoshi Masaaki Takezawa
1. Precise Determination of BaAl2O4 Cell and Certification of the Formation of Iron Bearing Solid Solution. By Hiroshi UCHIKAWA and Koichi TSUKIYAMA (
 1. Precise Determination of BaAl2O4 Cell and Certification of the Formation of Iron Bearing Solid Solution. By Hiroshi UCHIKAWA and Koichi TSUKIYAMA (Central Research Laboratory, Onoda Cement Co., Ltd.,
1. Precise Determination of BaAl2O4 Cell and Certification of the Formation of Iron Bearing Solid Solution. By Hiroshi UCHIKAWA and Koichi TSUKIYAMA (Central Research Laboratory, Onoda Cement Co., Ltd.,
TiO2ナノ粒子の部分硫化による可視光応答性光触媒の開発
 2002 CdS, ZnS by CdS CdS, ZnS CdS CdS, ZnS 52 36 15 14 14 10 10 10 3 3 (a) (b) (c) (d) (e) (f) (g) (h) ev, nm 1240 nm TiO 2 =3.2eV (= 390nm) (a) (b) (c)pt 1 Fe 2 O 3 2.2 TiO 2 (rutile) 3.0 Cu
2002 CdS, ZnS by CdS CdS, ZnS CdS CdS, ZnS 52 36 15 14 14 10 10 10 3 3 (a) (b) (c) (d) (e) (f) (g) (h) ev, nm 1240 nm TiO 2 =3.2eV (= 390nm) (a) (b) (c)pt 1 Fe 2 O 3 2.2 TiO 2 (rutile) 3.0 Cu
 19) Pearson, J., Southam, G. G. and Holley, R. A.: Survival and transport of bacteria in egg washwater. Appl. Environ. Microbiol., 53, 2060-2065 (1987). 20) Poovaiah, B. W.: Role of calcium in prolonging
19) Pearson, J., Southam, G. G. and Holley, R. A.: Survival and transport of bacteria in egg washwater. Appl. Environ. Microbiol., 53, 2060-2065 (1987). 20) Poovaiah, B. W.: Role of calcium in prolonging
untitled
 (a) (b) (c) (d) (e) (f) (g) (f) (a), (b) 1 He Gleiter 1) 5-25 nm 1/2 Hall-Petch 10 nm Hall-Petch 2) 3) 4) 2 mm 5000% 5) 1(e) 20 µm Pd, Zr 1(f) Fe 6) 10 nm 2 8) Al-- 1,500 MPa 9) 2 Fe 73.5 Si 13.5 B 9 Nb
(a) (b) (c) (d) (e) (f) (g) (f) (a), (b) 1 He Gleiter 1) 5-25 nm 1/2 Hall-Petch 10 nm Hall-Petch 2) 3) 4) 2 mm 5000% 5) 1(e) 20 µm Pd, Zr 1(f) Fe 6) 10 nm 2 8) Al-- 1,500 MPa 9) 2 Fe 73.5 Si 13.5 B 9 Nb
 * * ** * 507-0071 10-6-29 ** 755-8510 1985 Evaluation of Physico-Chemical Properties of Magnesium Oxide Masaaki Haneda*, Kiyotaka Kato*, Shouji Sakai** * Advanced Ceramics Research Center, Nagoya Institute
* * ** * 507-0071 10-6-29 ** 755-8510 1985 Evaluation of Physico-Chemical Properties of Magnesium Oxide Masaaki Haneda*, Kiyotaka Kato*, Shouji Sakai** * Advanced Ceramics Research Center, Nagoya Institute
研究成果報告書
 EL EL 17% 1.5 (F. Li, et al. Org. Elect., Vol. 8, 635, 2007 ) (S.Hore, et al., Sol. Energy Mater. Sol. Cells, pp. Vol. 90, 1176, 2006) (C. Haase et al., Proc. of SPIE, Vol. 6645, 66450W, 2007) (T. Fukuda
EL EL 17% 1.5 (F. Li, et al. Org. Elect., Vol. 8, 635, 2007 ) (S.Hore, et al., Sol. Energy Mater. Sol. Cells, pp. Vol. 90, 1176, 2006) (C. Haase et al., Proc. of SPIE, Vol. 6645, 66450W, 2007) (T. Fukuda
1 2 2 (Dielecrics) Maxwell ( ) D H
 2003.02.13 1 2 2 (Dielecrics) 4 2.1... 4 2.2... 5 2.3... 6 2.4... 6 3 Maxwell ( ) 9 3.1... 9 3.2 D H... 11 3.3... 13 4 14 4.1... 14 4.2... 14 4.3... 17 4.4... 19 5 22 6 THz 24 6.1... 24 6.2... 25 7 26
2003.02.13 1 2 2 (Dielecrics) 4 2.1... 4 2.2... 5 2.3... 6 2.4... 6 3 Maxwell ( ) 9 3.1... 9 3.2 D H... 11 3.3... 13 4 14 4.1... 14 4.2... 14 4.3... 17 4.4... 19 5 22 6 THz 24 6.1... 24 6.2... 25 7 26
Web Stamps 96 KJ Stamps Web Vol 8, No 1, 2004
 The Journal of the Japan Academy of Nursing Administration and Policies Vol 8, No 1, pp 43 _ 57, 2004 The Literature Review of the Japanese Nurses Job Satisfaction Research Which the Stamps-Ozaki Scale
The Journal of the Japan Academy of Nursing Administration and Policies Vol 8, No 1, pp 43 _ 57, 2004 The Literature Review of the Japanese Nurses Job Satisfaction Research Which the Stamps-Ozaki Scale
untitled
 1 / 37 5-4 6.1 1 2 / 37 1 (1) FePt AuAg (2) CdSe ZnSXY 2 O 3 X X : ZnO (3) SiO 2 (4) (5) 2 1 3 / 37 1 (1) FePt AuAg (2) CdSe ZnSXY 2 O 3 X X : ZnO (3) SiO 2 (4) (5) 3 4 / 37 1Tbits/cm 2 HD FePt FePt 110nm
1 / 37 5-4 6.1 1 2 / 37 1 (1) FePt AuAg (2) CdSe ZnSXY 2 O 3 X X : ZnO (3) SiO 2 (4) (5) 2 1 3 / 37 1 (1) FePt AuAg (2) CdSe ZnSXY 2 O 3 X X : ZnO (3) SiO 2 (4) (5) 3 4 / 37 1Tbits/cm 2 HD FePt FePt 110nm
 Time and Temperature Dependence of Recovery Behavior of Residual Strains in Largely Deformed Glassy Poly (Methyl Methacrylate) by Shin'ya YOSHIOKA*, Ryoji NAKAGAWA** and Yukuo NANZAI* Time and temperature
Time and Temperature Dependence of Recovery Behavior of Residual Strains in Largely Deformed Glassy Poly (Methyl Methacrylate) by Shin'ya YOSHIOKA*, Ryoji NAKAGAWA** and Yukuo NANZAI* Time and temperature
Application of Solid Electrolyte Sensors to Hot Corrosion Studies Nobuo Otsuka* *Iron & Steel Research Laboratories, Sumitomo Metal Industries, Ltd. C
 Application of Solid Electrolyte Sensors to Hot Corrosion Studies Nobuo Otsuka* *Iron & Steel Research Laboratories, Sumitomo Metal Industries, Ltd. Chemical/electrochemical aspects of hot corrosion of
Application of Solid Electrolyte Sensors to Hot Corrosion Studies Nobuo Otsuka* *Iron & Steel Research Laboratories, Sumitomo Metal Industries, Ltd. Chemical/electrochemical aspects of hot corrosion of
Frontier Simulation Software for Industrial Science
 PACS-CS FIRST 2005 2005 2 16 17 2 28 2 17 2 28 3 IT IT H14~H16 CHASE CHASE-3PT Protein Protein-DF ABINIT-MP 17 2 28 4 CMOS Si-CMOS CMOS-LSI CMOS ATP 10nm 17 2 28 5 17 2 28 6 CMOS CMOS-LSI LSI 90nm CMOS
PACS-CS FIRST 2005 2005 2 16 17 2 28 2 17 2 28 3 IT IT H14~H16 CHASE CHASE-3PT Protein Protein-DF ABINIT-MP 17 2 28 4 CMOS Si-CMOS CMOS-LSI CMOS ATP 10nm 17 2 28 5 17 2 28 6 CMOS CMOS-LSI LSI 90nm CMOS
コロイド化学と界面化学
 x 25 1 kg 1 kg = 1 l mmol dm -3 ----- 1000 mg CO 2 -------------------------------------250 mg Li + --------------------------------1 mg Sr 2+ -------------------- 10
x 25 1 kg 1 kg = 1 l mmol dm -3 ----- 1000 mg CO 2 -------------------------------------250 mg Li + --------------------------------1 mg Sr 2+ -------------------- 10
R927清水信彦様.indd
 Special Issue CFRP 455-8502 9 1 Development Status of Carbon Fiber Reinforced Plastics Nobuhiko SHIMIZU Automotive Center, Toray Industries, Inc., 9-1 Oe-cho, Minato-ku, Nagoya, Aichi 455-8502 Received
Special Issue CFRP 455-8502 9 1 Development Status of Carbon Fiber Reinforced Plastics Nobuhiko SHIMIZU Automotive Center, Toray Industries, Inc., 9-1 Oe-cho, Minato-ku, Nagoya, Aichi 455-8502 Received
PALL NEWS vol.126 November 2017
 PALL NEWS November 2017 Vol.126 PALL NEWS vol.126 November 2017 NEW =2000 9660 41.4 MPa 24 MPa NFPA T2.06.01 R2-2001 CAT C/90/* (1x10 6 0-28 MPa 1x10 6 29 120 C 60 C 450 Pa 340 Pa 1 MPa JIS B 8356-3/ISO
PALL NEWS November 2017 Vol.126 PALL NEWS vol.126 November 2017 NEW =2000 9660 41.4 MPa 24 MPa NFPA T2.06.01 R2-2001 CAT C/90/* (1x10 6 0-28 MPa 1x10 6 29 120 C 60 C 450 Pa 340 Pa 1 MPa JIS B 8356-3/ISO
hv (%) (nm) 2
 HOLLOW CATHODE LAMPS hv 1 2 1 8 5 3 4 6 (%) 6 4 7 8 2 16 2 24 28 32 36 4 44 (nm) 2 1 328.7 346.5 Ag 47-47NB(AG) 338.28 1 2 Re 75-75NB(RE) 346.47 2 25 39.27 343.49 Al 13-13NB(AL) 396.15 1 2 Rh 45-45NB(RH)
HOLLOW CATHODE LAMPS hv 1 2 1 8 5 3 4 6 (%) 6 4 7 8 2 16 2 24 28 32 36 4 44 (nm) 2 1 328.7 346.5 Ag 47-47NB(AG) 338.28 1 2 Re 75-75NB(RE) 346.47 2 25 39.27 343.49 Al 13-13NB(AL) 396.15 1 2 Rh 45-45NB(RH)
I A-7 CNT 27 A A A A A B A,C,D A B C D ( ) I A-8 Ce:YIG 31 A A,B A B JST I A-9 Cr 2 O 3 35 I A-10 CuO 39 A B A A A B I A-11 Co BiCoO 3 43 A A,B A A A
 24 12 13 ( ) I-A 9:50 9:55 9:55 10:50 12:20 13:50 TL-1 1 14:00 15:00 OL-2 7 JST CREST I-B I A 15:10 16:05 16:05 17:50 10F S10A I A-3 X 11 A B B B A B I A-4 X 15 A A I A-5 X 19 A A I A-6 X 23 A A i I A-7
24 12 13 ( ) I-A 9:50 9:55 9:55 10:50 12:20 13:50 TL-1 1 14:00 15:00 OL-2 7 JST CREST I-B I A 15:10 16:05 16:05 17:50 10F S10A I A-3 X 11 A B B B A B I A-4 X 15 A A I A-5 X 19 A A I A-6 X 23 A A i I A-7
42 1 Fig. 2. Li 2 B 4 O 7 crystals with 3inches and 4inches in diameter. Fig. 4. Transmission curve of Li 2 B 4 O 7 crystal. Fig. 5. Refractive index
 MEMOIRS OF SHONAN INSTITUTE OF TECHNOLOGY Vol. 42, No. 1, 2008 Li 2 B 4 O 7 (LBO) *, ** * ** ** Optical Scatterer and Crystal Growth Technology of LBO Single Crystal For Development with Optical Application
MEMOIRS OF SHONAN INSTITUTE OF TECHNOLOGY Vol. 42, No. 1, 2008 Li 2 B 4 O 7 (LBO) *, ** * ** ** Optical Scatterer and Crystal Growth Technology of LBO Single Crystal For Development with Optical Application
PowerPoint Presentation
 / 2008/04/04 Ferran Salleras 1 2 40Gb/s 40Gb/s PC QD PC: QD: e.g. PCQD PC/QD 3 CP-ON SP T CP-OFF PC/QD-SMZ T ~ps, 40Gb/s ~100fJ T CP-ON CP-OFF 500µm500µm Photonic Crystal SMZ K. Tajima, JJAP, 1993. Control
/ 2008/04/04 Ferran Salleras 1 2 40Gb/s 40Gb/s PC QD PC: QD: e.g. PCQD PC/QD 3 CP-ON SP T CP-OFF PC/QD-SMZ T ~ps, 40Gb/s ~100fJ T CP-ON CP-OFF 500µm500µm Photonic Crystal SMZ K. Tajima, JJAP, 1993. Control
9) H. SCHMCLZRIED: Z. Elektrochem. 66 (l%1) p ) W. D. KINGERY et al.: J. Am. Chem. Soc., 42 (1959), p ) F. HUND: Z. Phys. Chem., 199 (195
 9) H. SCHMCLZRIED: Z. Elektrochem. 66 (l%1) p. 572 10) W. D. KINGERY et al.: J. Am. Chem. Soc., 42 (1959), p.394 11) F. HUND: Z. Phys. Chem., 199 (1952), p. 142 12) J. F. ELLIOTT and M. GLEISER: " Thermochemistry
9) H. SCHMCLZRIED: Z. Elektrochem. 66 (l%1) p. 572 10) W. D. KINGERY et al.: J. Am. Chem. Soc., 42 (1959), p.394 11) F. HUND: Z. Phys. Chem., 199 (1952), p. 142 12) J. F. ELLIOTT and M. GLEISER: " Thermochemistry
技術会議資料
 La-Mg-Ni 2 H 2 O e - e - H OH - H + e - H H 2 O OH - H + e - O H O Ni Ni O H O H 3 La, Pr, Nd, etc. Ni, Co, Mn, Al, etc. 0 0.2 0.4 0.6 0.8 1 H/M Equilibrium Hydrogen Pressure MPa (MPa) 10 1 0.1 0.01 0.001
La-Mg-Ni 2 H 2 O e - e - H OH - H + e - H H 2 O OH - H + e - O H O Ni Ni O H O H 3 La, Pr, Nd, etc. Ni, Co, Mn, Al, etc. 0 0.2 0.4 0.6 0.8 1 H/M Equilibrium Hydrogen Pressure MPa (MPa) 10 1 0.1 0.01 0.001
Program4.dvi
 12 3 ( ) 10:00 10:05 III I 10:05 11:05 III I-3 I-22 1 3 11:20 12:30 III TL-1 1 / 13:30 14:30 III OL-2 5 A,B ( A, B CREST) II 14:45 16:05 III II-23 II-48 1 3 I 16:05 18:20 I, II I-3 MgAl 2 O 4 :Eu 2 O 3
12 3 ( ) 10:00 10:05 III I 10:05 11:05 III I-3 I-22 1 3 11:20 12:30 III TL-1 1 / 13:30 14:30 III OL-2 5 A,B ( A, B CREST) II 14:45 16:05 III II-23 II-48 1 3 I 16:05 18:20 I, II I-3 MgAl 2 O 4 :Eu 2 O 3
untitled
 213 74 AlGaN/GaN Influence of metal material on capacitance for Schottky-gated AlGaN/GaN 1, 2, 1, 2, 2, 2, 2, 2, 2, 2, 1, 1 1 AlGaN/GaN デバイス ① GaNの優れた物性値 ② AlGaN/GaN HEMT構造 ワイドバンドギャップ半導体 (3.4eV) 絶縁破壊電界が大きい
213 74 AlGaN/GaN Influence of metal material on capacitance for Schottky-gated AlGaN/GaN 1, 2, 1, 2, 2, 2, 2, 2, 2, 2, 1, 1 1 AlGaN/GaN デバイス ① GaNの優れた物性値 ② AlGaN/GaN HEMT構造 ワイドバンドギャップ半導体 (3.4eV) 絶縁破壊電界が大きい
Key Words: average behavior, upper and lower bounds, Mori-Tanaka theory, composites, polycrystals
 Key Words: average behavior, upper and lower bounds, Mori-Tanaka theory, composites, polycrystals (Q)1=C1(E)1, (0)2=C2(E)2 (4) QQQ= fi(o)1+f2(2+(1-fi-f2)(o)m E=flIE1+12(6)2+(1-fl-f2)(E)D (5a) (5b) (E)i=1E/D+-y,
Key Words: average behavior, upper and lower bounds, Mori-Tanaka theory, composites, polycrystals (Q)1=C1(E)1, (0)2=C2(E)2 (4) QQQ= fi(o)1+f2(2+(1-fi-f2)(o)m E=flIE1+12(6)2+(1-fl-f2)(E)D (5a) (5b) (E)i=1E/D+-y,
untitled
 TOF ENMA JAEA-RMS) TOF Pre-scission JAERI-RMS (m-state 16 O + 27 Al 150MeV d TOF Nucl. Phys. A444, 349-364 (1985). l = m d Pre-scission Scission 10-19 (Post_scission) (Pre-scission) Proton_fission Alpha_fission
TOF ENMA JAEA-RMS) TOF Pre-scission JAERI-RMS (m-state 16 O + 27 Al 150MeV d TOF Nucl. Phys. A444, 349-364 (1985). l = m d Pre-scission Scission 10-19 (Post_scission) (Pre-scission) Proton_fission Alpha_fission
第122号(A4、E)(4C)/3 宇野ほか
 trans Development of high performance heat resistant transparent polyimides based on trans-,,, -cyclopentanetetracarboxylic dianhydride Takaaki Uno Takashi Okada Igor Rozhanskii Toshimitsu Kikuchi Kohei
trans Development of high performance heat resistant transparent polyimides based on trans-,,, -cyclopentanetetracarboxylic dianhydride Takaaki Uno Takashi Okada Igor Rozhanskii Toshimitsu Kikuchi Kohei
JAJP
 Agilent 7500ce ORS ICP-MS Glenn Woods Agilent Technologies Ltd. 5500 Lakeside, Cheadle Royal Business Park Stockport UK Agilent 7500ce ICP-MS 5 7500ce (ORS) 1 ORS 7500ce ORS ICP-MS ( ) 7500 ICP-MS (27.12
Agilent 7500ce ORS ICP-MS Glenn Woods Agilent Technologies Ltd. 5500 Lakeside, Cheadle Royal Business Park Stockport UK Agilent 7500ce ICP-MS 5 7500ce (ORS) 1 ORS 7500ce ORS ICP-MS ( ) 7500 ICP-MS (27.12
研究室ガイダンス(H28)福山研.pdf
 1 2 3 4 5 4 He M. Roger et al., JLTP 112, 45 (1998) A.F. Andreev and I.M. Lifshitz, Sov. Phys. JETP 29, 1107 (1969) Born in 2004 (hcp 4 He) E. Kim and M.H.W. Chan, Nature 427, 225 (2004); Science 305,
1 2 3 4 5 4 He M. Roger et al., JLTP 112, 45 (1998) A.F. Andreev and I.M. Lifshitz, Sov. Phys. JETP 29, 1107 (1969) Born in 2004 (hcp 4 He) E. Kim and M.H.W. Chan, Nature 427, 225 (2004); Science 305,
スライド 1
 Matsuura Laboratory SiC SiC 13 2004 10 21 22 H-SiC ( C-SiC HOY Matsuura Laboratory n E C E D ( E F E T Matsuura Laboratory Matsuura Laboratory DLTS Osaka Electro-Communication University Unoped n 3C-SiC
Matsuura Laboratory SiC SiC 13 2004 10 21 22 H-SiC ( C-SiC HOY Matsuura Laboratory n E C E D ( E F E T Matsuura Laboratory Matsuura Laboratory DLTS Osaka Electro-Communication University Unoped n 3C-SiC
磁気測定によるオーステンパ ダクタイル鋳鉄の残留オーステナイト定量
 33 Non-destructive Measurement of Retained Austenite Content in Austempered Ductile Iron Yoshio Kato, Sen-ichi Yamada, Takayuki Kato, Takeshi Uno Austempered Ductile Iron (ADI) 100kg/mm 2 10 ADI 10 X ADI
33 Non-destructive Measurement of Retained Austenite Content in Austempered Ductile Iron Yoshio Kato, Sen-ichi Yamada, Takayuki Kato, Takeshi Uno Austempered Ductile Iron (ADI) 100kg/mm 2 10 ADI 10 X ADI
第1章 溶接法および機器
 670 ) ) ) ) 2.1 ) 42 671 Sn T m K. T m. T m. T m 2.2 JIS BAl BPd Al-Si Al-Mn KAlF -K AlF H O Ni 43 672 Sn-In Zn EU RoHS ) Pb Cd Hg JIS Z ( ) ) ) 3.1 44 673. MO HX MX H O M X (d) Young sf lf cos sl sf lf
670 ) ) ) ) 2.1 ) 42 671 Sn T m K. T m. T m. T m 2.2 JIS BAl BPd Al-Si Al-Mn KAlF -K AlF H O Ni 43 672 Sn-In Zn EU RoHS ) Pb Cd Hg JIS Z ( ) ) ) 3.1 44 673. MO HX MX H O M X (d) Young sf lf cos sl sf lf
光学
 Control of Refractive Indices of Optical Polymers: Theoretical Prediction and Molecular Design Method Shinji ANDO The fundamental theory and a method to predict the refractive indices and dispersions of
Control of Refractive Indices of Optical Polymers: Theoretical Prediction and Molecular Design Method Shinji ANDO The fundamental theory and a method to predict the refractive indices and dispersions of
1 d 6 L S p p p p-d d 10Dq 1 ev p-d d 70 % 1: NiO [3] a b CI c [5] NiO Ni [ 1(a)] Ni 2+ d 8 d 7 d 8 + hν d 7 + e d 7 1(b) d 7 p Ni 2+ t 3 2g t3 2g e2
![1 d 6 L S p p p p-d d 10Dq 1 ev p-d d 70 % 1: NiO [3] a b CI c [5] NiO Ni [ 1(a)] Ni 2+ d 8 d 7 d 8 + hν d 7 + e d 7 1(b) d 7 p Ni 2+ t 3 2g t3 2g e2 1 d 6 L S p p p p-d d 10Dq 1 ev p-d d 70 % 1: NiO [3] a b CI c [5] NiO Ni [ 1(a)] Ni 2+ d 8 d 7 d 8 + hν d 7 + e d 7 1(b) d 7 p Ni 2+ t 3 2g t3 2g e2](/thumbs/93/111390883.jpg) 3 3.1 3.1.1 - d s p d 3d d d d d d d 10 23 d d 1954 [1] d d 1970 I-II-VI 2 II-VI II 1:1 I III CuAlS 2 Al 3+ d 5 Fe 3+ d 5 S 3p d 6 L L [2] configuration interaction: CI d 5 1 1 d 6 L S p p p p-d d 10Dq
3 3.1 3.1.1 - d s p d 3d d d d d d d 10 23 d d 1954 [1] d d 1970 I-II-VI 2 II-VI II 1:1 I III CuAlS 2 Al 3+ d 5 Fe 3+ d 5 S 3p d 6 L L [2] configuration interaction: CI d 5 1 1 d 6 L S p p p p-d d 10Dq
Al-Si系粉末合金の超塑性
 Superplasticity of Al-Si P/M Alloys Satoru Ishihara, Mikio Kondoh, Kazuhiko Itoh Al-17Si-4.5Cu-0.5Mg-(06)Fe-(02)Mm ( Mm : ) (i) (ii) Si(iii) Fe (iv) Mm Al-17Si-4.5Cu-0.5Mg500 10 2 s 1 520 110 1 s 1 Si
Superplasticity of Al-Si P/M Alloys Satoru Ishihara, Mikio Kondoh, Kazuhiko Itoh Al-17Si-4.5Cu-0.5Mg-(06)Fe-(02)Mm ( Mm : ) (i) (ii) Si(iii) Fe (iv) Mm Al-17Si-4.5Cu-0.5Mg500 10 2 s 1 520 110 1 s 1 Si
„´™Ÿ/’£flö
 48 144 2006 206-213 Journal of the Combustion Society of Japan Vol. 48 No. 144 (2006) 206-213 ORGNAL PAPER * * An Approach to Combustion Diagnostics of Premixed Flame by Chemiluminescence of OH * and CH
48 144 2006 206-213 Journal of the Combustion Society of Japan Vol. 48 No. 144 (2006) 206-213 ORGNAL PAPER * * An Approach to Combustion Diagnostics of Premixed Flame by Chemiluminescence of OH * and CH
物理化学I-第12回(13).ppt
 I- 12-1 11 11.1 2Mg(s) + O 2 (g) 2MgO(s) [Mg 2+ O 2 ] Zn(s) + Cu 2+ (aq) Zn 2+ (aq) + Cu(s) - 2Mg(s) 2Mg 2+ (s) + 4e +) O 2 (g) + 4e 2O 2 (s) 2Mg(s) + O 2 (g) 2MgO(s) Zn(s) Zn 2+ (aq) + 2e +) Cu 2+ (aq)
I- 12-1 11 11.1 2Mg(s) + O 2 (g) 2MgO(s) [Mg 2+ O 2 ] Zn(s) + Cu 2+ (aq) Zn 2+ (aq) + Cu(s) - 2Mg(s) 2Mg 2+ (s) + 4e +) O 2 (g) + 4e 2O 2 (s) 2Mg(s) + O 2 (g) 2MgO(s) Zn(s) Zn 2+ (aq) + 2e +) Cu 2+ (aq)
pp 427 438 2006 Dimensional Change Card Sort ****** ** 2005 9 30 2004 8 2002 4 Zelazo, P. D., Carter, A., Reznick, J. S. & Frye, D. 1997 10 2003 Zelaz
 Title 幼 児 の 実 行 機 能 の 発 達 過 程 : Dimensional Change C を 用 いたルールの 理 解 とその 使 用 に 関 する 検 討 Author(s) 浮 穴, 寿 香 ; 橋 本, 創 一 ; 出 口, 利 定 Citation 東 京 学 芸 大 学 紀 要. 総 合 教 育 科 学 系, 57: 427-438 Issue Date 2006-02-00
Title 幼 児 の 実 行 機 能 の 発 達 過 程 : Dimensional Change C を 用 いたルールの 理 解 とその 使 用 に 関 する 検 討 Author(s) 浮 穴, 寿 香 ; 橋 本, 創 一 ; 出 口, 利 定 Citation 東 京 学 芸 大 学 紀 要. 総 合 教 育 科 学 系, 57: 427-438 Issue Date 2006-02-00
渡辺(2309)_渡辺(2309)
 [ 29 p. 241-247 (2011)] ** *** ** ** Development of a nickel-based filler metal containing a small amount of silicon by WATANABE Takehiko, WAKATSUKI Ken, YANAGISAWA Atsusi and SASAKI Tomohiro Authors tried
[ 29 p. 241-247 (2011)] ** *** ** ** Development of a nickel-based filler metal containing a small amount of silicon by WATANABE Takehiko, WAKATSUKI Ken, YANAGISAWA Atsusi and SASAKI Tomohiro Authors tried
Degradation Mechanism of Ethylene-propylene-diene Terpolymer by Ozone in Aqueous Solution Satoshi MIWA 1 *, 2, Takako KIKUCHI 1, 2, Yoshito OHTAKE 1 a
 Degradation Mechanism of Ethylene-propylene-diene Terpolymer by zone in Aqueous Solution Satoshi MIWA 1, 2, Takako KIKUCHI 1, 2, Yoshito TAKE 1 and Keiji TANAKA 2 ( 1 Chemicals Evaluation and Research
Degradation Mechanism of Ethylene-propylene-diene Terpolymer by zone in Aqueous Solution Satoshi MIWA 1, 2, Takako KIKUCHI 1, 2, Yoshito TAKE 1 and Keiji TANAKA 2 ( 1 Chemicals Evaluation and Research
Feature Article Earozoru Kenkyu, 26 1, Preparation of Nanoparticles by Ultrasonic Spray Pyrolysis 1 Takashi OGI 1 and Kikuo OKUYAMA 1 Rec
 Feature Article Earozoru Kenkyu, 26, 36 4 20 Preparation of Nanoparticles by Ultrasonic Spray Pyrolysis Takashi OGI and Kikuo OKUYAMA Received 2 August 200 Accepted 29 September 200 Abstract In this review,
Feature Article Earozoru Kenkyu, 26, 36 4 20 Preparation of Nanoparticles by Ultrasonic Spray Pyrolysis Takashi OGI and Kikuo OKUYAMA Received 2 August 200 Accepted 29 September 200 Abstract In this review,
C-2 NiS A, NSRRC B, SL C, D, E, F A, B, Yen-Fa Liao B, Ku-Ding Tsuei B, C, C, D, D, E, F, A NiS 260 K V 2 O 3 MIT [1] MIT MIT NiS MIT NiS Ni 3 S 2 Ni
![C-2 NiS A, NSRRC B, SL C, D, E, F A, B, Yen-Fa Liao B, Ku-Ding Tsuei B, C, C, D, D, E, F, A NiS 260 K V 2 O 3 MIT [1] MIT MIT NiS MIT NiS Ni 3 S 2 Ni C-2 NiS A, NSRRC B, SL C, D, E, F A, B, Yen-Fa Liao B, Ku-Ding Tsuei B, C, C, D, D, E, F, A NiS 260 K V 2 O 3 MIT [1] MIT MIT NiS MIT NiS Ni 3 S 2 Ni](/thumbs/89/99121913.jpg) M (emu/g) C 2, 8, 9, 10 C-1 Fe 3 O 4 A, SL B, NSRRC C, D, E, F A, B, B, C, Yen-Fa Liao C, Ku-Ding Tsuei C, D, D, E, F, A Fe 3 O 4 120K MIT V 2 O 3 MIT Cu-doped Fe3O4 NCs MIT [1] Fe 3 O 4 MIT Cu V 2 O 3
M (emu/g) C 2, 8, 9, 10 C-1 Fe 3 O 4 A, SL B, NSRRC C, D, E, F A, B, B, C, Yen-Fa Liao C, Ku-Ding Tsuei C, D, D, E, F, A Fe 3 O 4 120K MIT V 2 O 3 MIT Cu-doped Fe3O4 NCs MIT [1] Fe 3 O 4 MIT Cu V 2 O 3
Natural Convection Heat Transfer in a Horizontal Porous Enclosure with High Porosity Yasuaki SHIINA*4, Kota ISHIKAWA and Makoto HISHIDA Nuclear Applie
 Natural Convection Heat Transfer in a Horizontal Porous Enclosure with High Porosity Yasuaki SHIINA*4, Kota ISHIKAWA and Makoto HISHIDA Nuclear Applied Heat Technology Division, Japan Atomic Energy Agency,
Natural Convection Heat Transfer in a Horizontal Porous Enclosure with High Porosity Yasuaki SHIINA*4, Kota ISHIKAWA and Makoto HISHIDA Nuclear Applied Heat Technology Division, Japan Atomic Energy Agency,
振動充填燃料の粒子焼結試験実施計画書
 C JNCTJ8410 2004-006 2004 3 ( ) UPRISE Nd 250mL 0.2 mol Nd Nd (FP)DF DOBA DOiBA NN'-2--1.6 mol/l NN'- -1.7 mol/l NN'-1.5 mol/l DOBA 1mol/L 3.5mol/L 2 NN'--1.7 mol/l NN' -1.5 mol/l ZrRu Ce DF 150 100 Sr
C JNCTJ8410 2004-006 2004 3 ( ) UPRISE Nd 250mL 0.2 mol Nd Nd (FP)DF DOBA DOiBA NN'-2--1.6 mol/l NN'- -1.7 mol/l NN'-1.5 mol/l DOBA 1mol/L 3.5mol/L 2 NN'--1.7 mol/l NN' -1.5 mol/l ZrRu Ce DF 150 100 Sr


